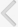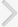INTRODUCTION
Interstitial cystitis (IC) is one of the most commonly seen chronic inflammatory diseases of the urinary bladderand is characterized by a wide range of symptoms, including pelvic or lower abdominal pain and irritative voiding symptoms such as urinary frequency, urgency, and nocturia [1-3]. Overlapping patterns of lower urinary tract symptoms and pelvic pain are common in patients with IC [4], and these overlapping patterns create challenges for clinical research and practice and warrant further investigation of their causes, diagnosis, and effective treatments.
The conditions of IC and overactive bladder (OAB) share irritative voiding symptoms [1,4]. Theoretically, then, anticholinergic drugs could be considered to provide an alternate mechanism of action, which could decrease these embarrassing symptoms. In practice, however, many studies have shown that most IC patients do not respond appropriately to treatment with anticholinergic medications, whereas OAB patients with benign prostatic hyperplasiarespond well [5,6]. Thus, we need to clarify the urodynamic findings and mechanismsin these two experimental conditions in animal models.
The OAB syndrome is defined as urinary urgency, with or without urge incontinence, that is usually accompanied by urinary frequency and nocturia [7]. In other words, this urgency is regarded as a core symptom in diagnosis, which may correspond to detrusor overactivity (DO) during the filling phase in human urodynamic study [7,8]. Assuming that nonvoiding contractions (NVCs) interpreted on the basis of simultaneous changes in intraabdominal pressure (IAP) in an animal model can be used as a substitute parameter for DO in humans, the rat model of chemical cystitis and bladder outlet obstruction (BOO) may allow us to study the pathophysiology of different responsesto anticholinergic drugs.
In this study, we investigated bladder function, with a special focus on the NVCs, in awake rats with chemical cystitis and BOO by use of simultaneous registrations of intravesical and intraabdominal pressures. In addition, we tested the effects of tolterodine on the NVCs in these models.
MATERIALS AND METHODS
Animals and Study Design
A total of 20 female Sprague-Dawley rats (250 to 295 g; Orient Bio Inc., Seongnam, Korea) were used in this study. All experimental animals and procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and were approved by the local animal ethics committee. The rats were maintained under standard laboratory conditions with 12:12 hours light:dark cycles and free access to food pellets and tap water. In eight rats, chemical cystitis was induced by intravesical instillation of HCl. Twelve rats were subjected to sham instillations or partial BOO. Four weeks after intravesical instillation or 2 weeks after partial BOO, cystometrograms were obtained in all unanesthetized, unrestrained rats in metabolic cages.
Induction of Cystitis
Rats were anesthetized with intraperitoneal ketamine (Ketamine, Yuhan Corp., Seoul, Korea; 75 mg/kg) and xylazine (Rompun, Bayer Korea Ltd., Seoul, Korea; 15 mg/kg intraperitoneally). Cystitis was induced by the intravesical instillation of 0.5 mL of 0.4 N HCl [9]. Through an abdominal incision under anesthesia, a 24-gauge needle with syringe was inserted into the dome of the bladder. After all of the urine was aspirated, HCl was instilled into the bladder lumen for 90 seconds. The same volume of normal saline was instilled in the sham group.
Procedures for Intravesical, Intraabdominal, and Intravenous Catheter Implantation
Three days before cystometry, catheterizations for simultaneous recordings of intravesical pressure (IVP) and IAP were performed as described previously [10]. Briefly, after anesthesia, a polyethylene catheter (PE-50, Becton Dickinson, Parsipanny, NJ, USA) with a cuff was implanted into the dome of the bladder through an abdominal incision. To record IAP, an abdominal balloon (Latex, Dawoo Medical, Incheon, Korea) around the cuff of a catheter tip was placed proximal to the bladder and was tied to another catheter with silk tie. To deliver the anticholinergic agent during awake cystometry, a polyethylene catheter (PE-10) was inserted into a femoral vein, which was filled with heparinized (30 IU) saline. These catheters were then tunnelled through the subcutaneous space and exited the back of the animal and were anchored to the skin of the back. After surgery, each rat was caged individually and maintained in the same manner.
Functional Evaluation
Cystometrograms were obtained in unanesthetized, unrestrained rats in metabolic cages. The indwelling catheter to the bladder was connected to a two-way valve that was connected via a T-tube to a pressure transducer (Research Grade Blood Pressure Transducer, Harvard Apparatus, Holliston, MA, USA) and a microinjection pump (PHD22/2000 pump, Harvard Apparatus). Another indwelling catheter connected to a fluid-filled abdominal balloon was connected to another pressure transducer to record the IAP. Micturition volumes were recorded by means of a fluid collector connected to a force displacement transducer (Research Grade Isometric Transducer; Harvard Apparatus). IVP, IAP, and micturition volumes were recorded continuously with Acq Knowledge 3.8.1 software and an MP150 data acquisition system (BIOPAC Systems, Goleta, CA, USA) at a sampling rate of 50 Hz. The mean values from three reproducible micturition cycles were used for evaluation. IAP was defined as the recorded balloon pressure minus the lowest balloon pressure in each voiding cycle. Detrusor pressure (DP) was defined as IVP minus IAP. The IVP rises during the filling phase were defined as increments of IVP that exceeded 2 cm-H2O from baseline, which was interpreted as DO if occurring without simultaneous similar changes in IAP and was interpretedas abdominal straining if occurring with simultaneous similar changes in IAP.
Investigated Urodynamic Parameters
Pressure- and volume-related parameters
These included basal pressure (BP; the lowest bladder pressure during filling), threshold pressure (TP; bladder pressure immediately before micturition), maximum pressure (MP; maximum bladder pressure during the micturition cycle), micturition volume (MV; volume of expelled urine), residual volume (RV; remaining urine after voiding), bladder capacity (BC; MV+RV), and micturition interval (MI; intervals between micturition contractions).
DO-related parameters during the filling phase
These included time of filling phase (interval from the initiation of infusion through the tube and the point immediately before the initiation of micturition), frequency of abdominal strainingper minute, frequency of DO per minute, and increased amplitude from base to peak of DO spike as IVP. These frequencies were calculated on the basis of the time of filling phase. After cystometry, the animals were sacrificed by cervical dislocation. The bladder and urethra were removed en bloc and separated at the level of the bladder neck, and the bladder was weighed.
Administration of Drug
During cystometry, room temperature saline was infused into the bladder at a rate of 10 mL.h-1. Micturitions during intravesical saline infusion served as baseline values. After 0.2 mL tolterodine (0.3 mg/kg) was injected intravenously, there was a 30-minute observation period.
Statistical Analysis
The results are given as mean values┬▒standard errors of the mean (SEMs). Normal distributions were confirmed by the Shapiro-Wilks' W test. One-way analysis of variance with Tukey's test was used to detect differencesin urodynamic parameters among the sham, cystitis, and BOO groups before drug administration, and paired testswere used to compare these parameters between before and after drug administration in the same group. All analyses were performed with GraphPad Prism, ver. 5.03, 2009 (GraphPad Software, San Diego, CA, USA). Statistical significance was considered at P<0.05, P<0.01, and P<0.001. All calculations were made based on n, which denoted the number of animals.
RESULTS
Comparison of Body Weights and the Ratio of Bladder to Body Weight
There wereno significant differences among the sham (266.7┬▒6.7 g), cystitis (281.7┬▒4.4 g), and BOO (261.0┬▒6.0 g) groups in body weight at 2 weeks after intravesical instillations or obstruction surgery. However, the ratio of urinary bladder weight to body weight significantly increased in the BOO group (1.00┬▒0.12) compared with the sham (0.43┬▒0.02) andcystitis (0.45┬▒0.03) groups. There was no significant difference between the sham and cystitis groups in that ratio (Fig. 1A).
Comparison of Pressure-Related Parameters among the Three Groups and Effects of Tolterodine
The BOO group showed increased BP and TP by DP compared with thecystitis and sham groups, but there were no significant differences in those values between the cystitis and sham groups. There were no significant differences in MP by DP among the three groups (Fig. 1B-D).
After the administration of tolterodine, the cystitis and BOO groups showed decreased MP by DP compared withthe values before medication (Fig. 1D). The BOO group showed decreased TP by DP, whereas the cystitis group showed no changes in this variable after medication. BP by DP did not change significantly in either group with medication (Fig. 1B, C).
Comparison of Volume-Related Parameters among the Three Groups and Effects of Tolterodine
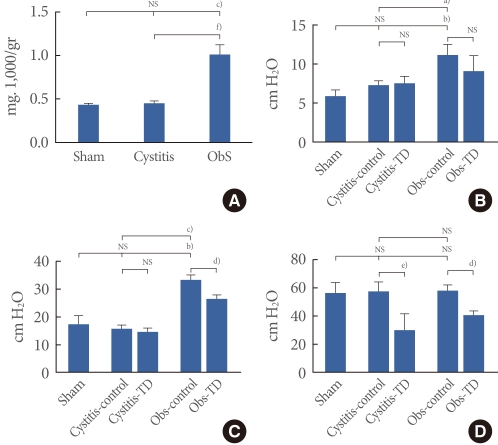
There were no significant differences in any volume-related parameters before medication, including BC, MV, RV, and MI, among thesham, cystitis, and BOO groups (Fig. 2).
There were no significant changes in BC or MI after the administration of tolterodine in either cystitisor BOO rats. Also, the cystitis group showed no changes in MV after medication, whereas values decreased in the BOO group. As for RV, the cystitis group also showed no changes despite higher values in the BOO group after medication (Fig. 2).
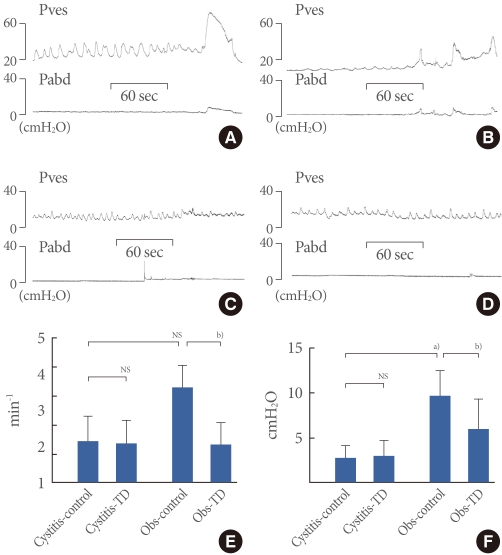
Comparison of DO-Related Parameters between the Sham and Cystitis Groups and Effects of Toleterodine
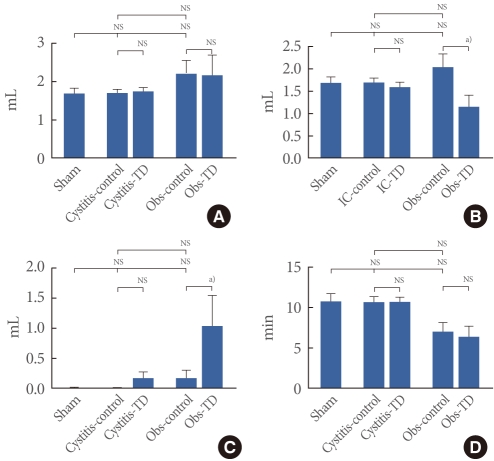
The cystitis group showed the DO in 50% (3 of 6 rats), which was not seen in the sham group. Before theadministration of tolterodine, the cystitis group showed lower DO pressure, without a difference in DO frequency, than did the BOO group. After the administration, the cystitis group showed no difference in DO frequency orpressure, although the BOO group showed decreased values forboth parameters (Figs. 3, 4).
DISCUSSION
Our study showed that tolterodine had no significant effect on urodynamic parameters, including pressure/volume and DO-related variables, except MP in a chronic IC model. Only MP showed a decrease with treatment without changes in DO frequency or pressure. This suggests that toleterodine may have an unfavorable effect on voiding without any effect on DO during the filling phase. In the BOO model, however, the drug resulted in adecrease inboth frequency and pressure of DO and some changes in pressure/volume parameters. The anticholinergic drug showed some effects on both the filling and voiding phases. IC is a chronic inflammatory disease of the bladder [1], but its etiology and pathogenesis remain obscure. It is characterized by irritative voiding symptoms and vague pain related to the bladder and shareswith OAB syndromeurinary urgency as a key symptom in diagnosis [1,4]. The urgency symptom is known to be correlated with the DO, which is defined as a urodynamic observation of involuntary detrusor contractions during the bladder-filling phase [7,8]. About 15% of patients with IC have been reported to have spontaneous DO as measured by urodynamic testing [11]. The treatment of this DO has incorporated the application of anticholinergic drugs, but many clinical reports have shownthat these drugs are ineffective in many patients with IC [5,6]. There havebeen few reports directly showing the effects of anticholinergic drugs on urodynamic results, including objective report of DO in an awake animal IC model. According to our results, DO was not affected by the anticholinergic drug in the IC model, unlike in the BOO model. Such study to find the cause of the ineffectiveness in an animal model is important to improve our understanding of these drugs and to find possible alternative treatments.
The uncertainty over the benefit of anticholinergic drugs in IC appears to stem from two separate factors of unclear diagnostic criteria and etiology or pathophysiology. In the diagnosis of IC, after ruling out other obvious pathology and overlapping syndromes through an extensive and specific series of tests [12], the exclusion of other often confused diseases is still encouraged, because there are no definitive diagnostic tests. Since the National Institute for Diabetes and Diseases of the Kidney (NIDDK) reported the diagnostic criteria for IC in 1990 [13], the definitions and terminology have undergone some changes. Different organizations have proposed different terms, such as painful bladder syndrome or bladder pain syndrome [14,15]. Enigmatic pain related to the bladder was emphasized more than the other symptoms in these reports [3,14]. However, because these small changes in diagnostic criteria can result in noticeably different patient groups being studied, we need to unify the terms or definitions and focus on how to delineate the patients accurately by use of accurate diagnostic criteria internationally. The heterogeneous characteristics of IC subgroups could be the starting obstacle in finding a solution to the ineffectiveness of anticholinergic drugs. It will be important to find a subgroup of IC patients who respond well to this drug or to determine how to use this drug as a palliative agent.
Second, several decades of research on IC has revealed that inflammation, as determined by the presence of cytokines, chemokines and mast cells, may have vital role in the pathogenesis of this disease [16,17]. Although it is suggested that increased permeability of or neuropathic pain in the bladder mucosa results in OAB through the activation of afferent reflex circuitry [18-20], our limited understanding of the fundamental mechanisms causing OAB in IC has continued to frustrate efforts aimed at its treatment. The detailed mechanism needs further elucidation.
Our proposed model of chronic chemical cystitis showed the characteristics of OAB in view of DO-related parameters. Our modelshowed DO in 50% of the rats, even with no increased BP, TP, or MP and no decreased BC, MV, RV, or MI compared with the control rats. These animals at 4 weeks did not show differences compared with animals at 2 weeks in our previous study [21]. Our model of BOO also showed characteristics similar to the results of previous studies with BOO. The BOO rats showed an increased ratio of bladder to body weight, increased BP, and increased TP, but no increases in BC, MI, or RV. After theadministration of tolterodine, the BOO model showed a decrease inTP, MP, and DO pressure/frequency and an increase in RV, corresponding to the original effects of that drug.
In the obstructed bladder, supersensitivity of the detrusor muscle secondary to denervation was found to play a predominant pathophysiologic role in OAB [22,23]. This has been suggested to be caused by bladder overdistention resulting from BOO [24]. Clinically, DO in men with OAB responds well to antimuscarinics, which is similar the results of our study [25]. An anticholinergic agent is a substance that blocks the acetylcholine of a neurotransmitter by competitive inhibition in neurons of the central and peripheral nervous system. This is known to reduce the effects mediated by acetylcholine on muscarinic receptors in those neurons [26]. We suspect that the different response between BOO and IC is caused by the basic difference in the pathophysiologic characteristics of the bladder in these two conditions, which needs to be elucidated further in future studies. Clinically, we also need further study to improve the treatment effect of anticholinergic drugs inIC patients with OAB.