Is the Brainstem Activation Different Between Healthy Young Male and Female Volunteers at Initiation of Voiding? A High Definition 7-Tesla Magnetic Resonance Imaging Study
Article information
Abstract
Purpose
Assessing brainstem function in humans through typical neuroimaging modalities has been challenging. Our objective was to evaluate brain and brainstem activation patterns during initiation of voiding in healthy males and females utilizing a 7 Tesla magnetic resonance imaging (MRI) scanner and a noninvasive brain-bladder functional MRI (fMRI) protocol.
Methods
Twenty healthy adult volunteers (10 males and 10 females) with no history of urinary symptoms were recruited. Each volunteer underwent a clinic uroflow and postvoid residual assessment and was asked to consume water prior to entering the scanner. Anatomical and diffusion tensor images were obtained first, followed by a blood oxygenation level dependent (BOLD) resting-state fMRI (rs-fMRI) during the empty bladder. Subjects indicated when they felt the urge to void, and a full bladder rs-fMRI was obtained. Once completed, the subjects began 5 voiding cycles, where the first 7.5 seconds of each voiding cycle was identified as “initiation of voiding.” BOLD activation maps were generated, and regions of interests with a t-value greater than 2.1 were deemed statistically significant.
Results
We present 5 distinct regions within the periaqueductal gray (PAG) and pontine micturition center (PMC) with statistically significant activation associated with an initiation of voiding in both men and women, 3 within the PAG and 2 within the PMC. Several additional areas in the brain also demonstrated activation as well. When comparing males to females, there was an overall lower BOLD activation seen in females throughout all regions, with the exception of the caudate lobe.
Conclusions
Our study effectively defines regions within the PAG and PMC involved in initiation of voiding in healthy volunteers. To our knowledge, this is the first study investigating differences between male and female brainstem activation utilizing an ultra-high definition 7T MRI.
INTRODUCTION
Despite significant progress in identifying and characterizing brain regions involved in lower urinary tract (LUT) control, imaging the human brainstem has remained challenging. More specifically, functional neuroimaging of the brainstem is particularly difficult due to its small size, neurophysiological noise, and limited resolution associated with typical 1.5 and 3T magnetic resonance imaging (MRI) studies. The use of ultra-high resolution 7-Tesla MRI scanners have provided improved visualization of complex subcortical structures, such as the brainstem. A higher magnetic field allows for increased spatial resolution, greater signal-to-noise ratio and submillimeter voxel sizes, yielding higher quality imaging of smaller structures deep within the brain in reasonable time frames [1]. There are multiple key structures involved in proper LUT function in the brainstem. One of the most important regions is the periaqueductal gray (PAG), which is believed to be crucial for interpreting information from the bladder and relaying it to cortical and subcortical areas of the brain [2]. Another widely studied region involved in LUT function is the pontine micturition center (PMC) [3]. Activation of this region initiates the micturition (bladder emptying) reflex, characterized by relaxation of the urethral sphincters and contraction of the detrusor muscle. With the PAG and PMC serving as the afferent and efferent branches of signaling respectively, these 2 regions have been shown to work together during typical voiding [4]. In contrast, the pontine storage center (PSC) is well known to contract the internal urethral sphincter to inhibit voiding [5]. Details concerning the current working model of brain-bladder circuits can be found in Fig. 1 [3,6-8].
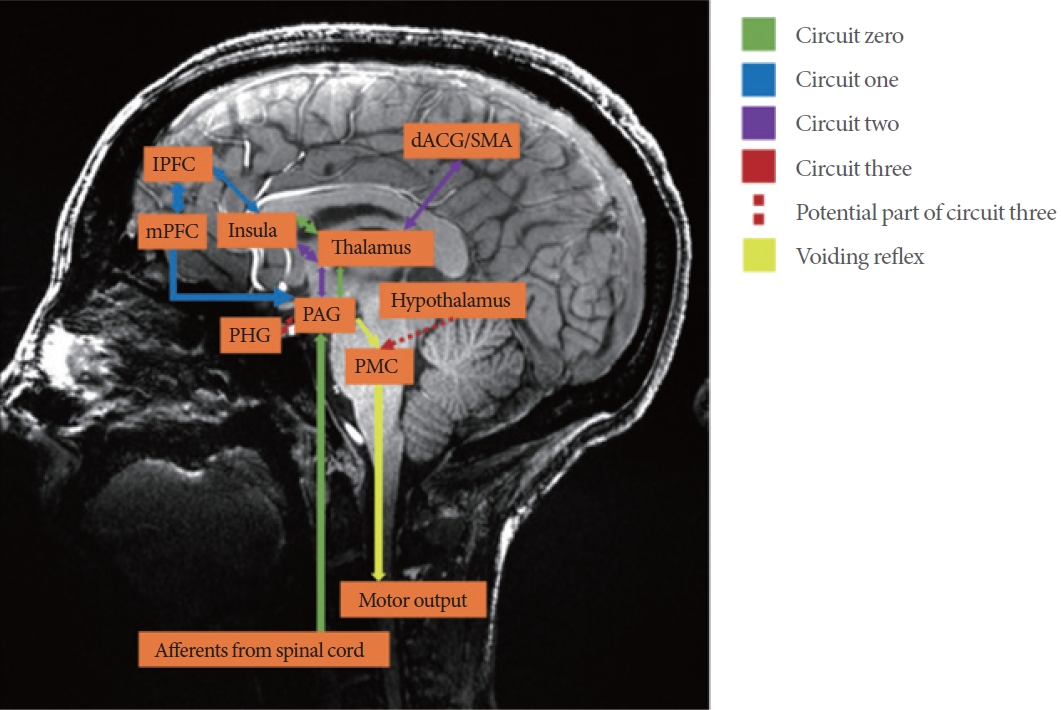
The 4 circuits involved in voiding with their approximate regions depicted on a sagittal image form one of our volunteers with 7-Tesla (7T) magnetic resonance imaging. The current leading theory on neural control of lower urinary tract (LUT) continence and voiding proposes that regions of interest in the brain and brainstem interact in 4 circuits [1,2-4]. Circuit zero involves the reception of afferent information from the bladder to the cortex and determines whether the bladder is full enough for voiding to occur. Circuit one is believed to be the social aspect of voiding and as such, involves the prefrontal cortex. Circuit 2 checks whether voluntary voiding is appropriate emotionally and involves the limbic system. Finally, circuit 3 confirms whether it is safe to void and involves the parahippocampal gyrus, the periaqueductal gray (PAG), and potentially the hypothalamus. IPFC, lateral prefrontal cortex; mPFC, medial profrontal cortex; PHG, parahippocampal gyrus; PMC, pontine micturition center; dACG, dorsal anterior cingulate gyrus; SMA, supplementary motor area.
Blood oxygenation level dependent (BOLD) fMRI leverages signals that appear as differing rates of oxygen consumption during neuronal activity than at rest to determine an active or inhibited region [9]. During neuronal activation, these brain regions receive higher rates of blood flow with more oxygenated hemoglobin due to increased metabolic demand [10]. Deoxygenated hemoglobin is more magnetic than oxygenated hemoglobin, which is resistant to magnetism (diamagnetic). As fMRI can detect the difference in oxygenated and deoxygenated hemoglobin [11], this phenomenon serves as a form of “magnetic” contrast for fMRI images by depicting brain regions that are active during specific tasks (an indirect measure of activation) [12]. Utilizing the 7T MRI, we are now able to investigate brainstem activation in specific regions during the task of voiding to better understand the complex pathways and their activation or inhibition.
The objective of this study is to evaluate the brain and specific brainstem activation involved in the initiation of voiding in healthy participants to better identify previously hypothesized regions of interest (ROIs) that are involved in LUT control. Specifically, we aim to identify the ‘switch’ from continence to voiding utilizing ultra-high resolution 7T MRI. Furthermore, we investigated the differences in activation patterns between male and female participants at the time of initiation (or attempt) of voiding.
MATERIALS AND METHODS
Eligible participants were screened and recruited following registration of our clinical trial (NCT04846387) and Institutional Review Board approval. Informed consent was obtained for each subject. Inclusion criteria consisted of healthy adult volunteers ( > 18 years of age) with postvoid residual (PVR) of < 20% bladder capacity along with an American Urology Association Symptom Score of < 7. Exclusion criteria included any history of genitourinary surgery or malignancy, any neurological disorder, prior history of seizures, pregnancy, or contraindication to MRI.
During the screening visit, participants arrived with a full bladder. Uroflow and PVR were performed to ensure continuation in the study. Participants were instructed to refrain from caffeinated drinks 2 hours prior to arrival and consume 500–750 mL of water roughly 20 minutes prior to the scan (based on their bladder capacity on uroflow and their preference). Subjects were given instructions to practice the urine voiding-holding task, which consisted of 5 cycles of 45 seconds each. The first 15 seconds of each cycle was reserved for voiding/attempting to void, followed by 30 seconds of voluntary urethral contraction preventing voiding; or the holding task (Fig. 2). Immediately prior to entering the MRI scan room, participants emptied their bladder and PVR was obtained. A 7-Tesla Siemens MAGNETOM Terra MRI scanner (Siemens Medical Solutions USA Inc., Malvern, PA, USA) was used for all image acquisition. Participants were instructed to perform the urine voiding-holding task as before for the first 4 cycles, and void to completion into an external catheter during the final voiding attempt (5th cycle), which was collected in a volumetric bag. A screen located within the MRI room visible to the participants displayed the current task and counted the length of time for the subject to complete. Additionally, technicians were able to communicate with the participants through the intercom system native to the MRI scanner. Voided volumes in the MRI scanner and PVR were recorded for each participant.
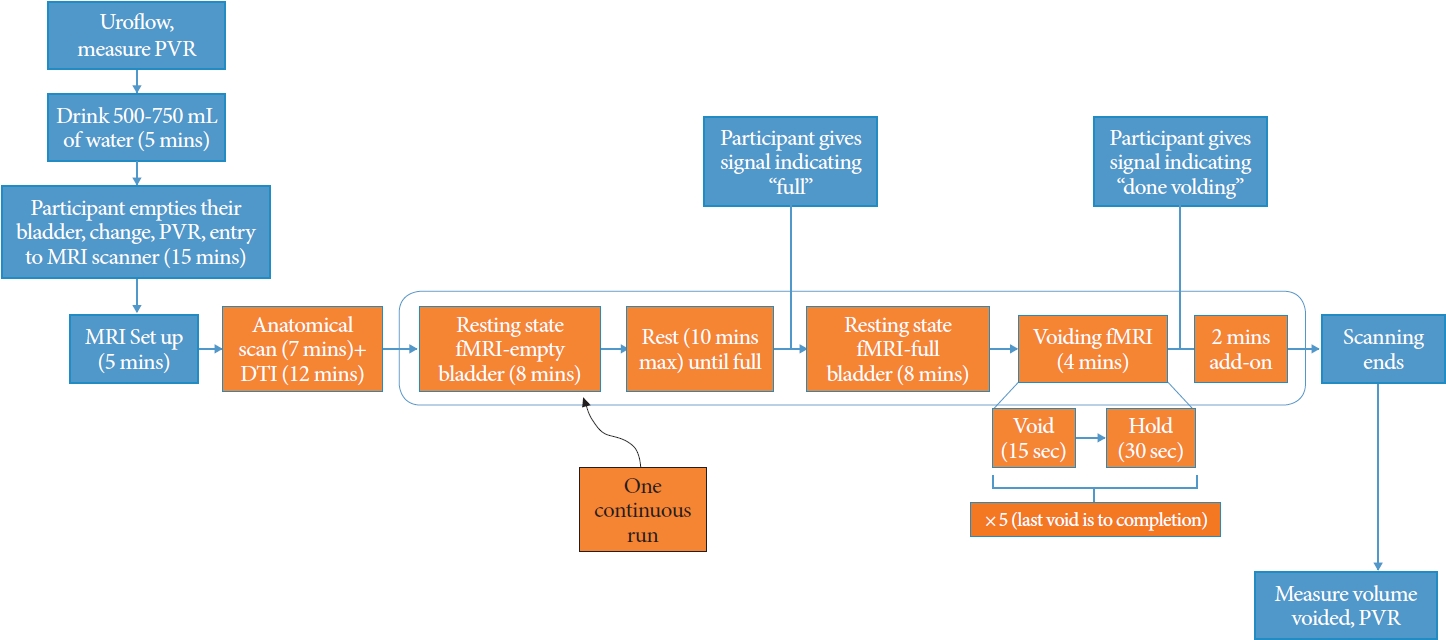
Study protocol for image acquisition. PVR, postvoid residual; MRI, magnetic resonance imaging; DTI, diffusion tensor imaging; fMRI, functional MRI.
Isotropic structural and functional magnetic resonance images were acquired. T1 MPRAGE anatomical images were obtained in the sagittal plane at a spatial resolution of 0.7 mm3 with a TR of 2,200 msec, TE of 2.95 msec and slice thickness of 0.7 mm. Functional scans produced a 1.7 mm3 isotropic array of axial images with a TR of 2,400 msec, TE of 24 ms and a corresponding slice thickness of 1.7 mm. Both anatomical and functional MRI BOLD maps of the brain and brainstem were created for the ROI during empty bladder at resting-state and with full bladder voiding (or attempt of voiding) tasks.
Authors are still planning to perform other analysis on the current data. Authors anticipate having all their analysis completed in 5 years. During this time authors would welcome any requests to access the data.
Data and Statistical Analysis
Functional MRI data analysis was performed with AFNI (Analysis of Functional NeuroImages). Anatomical and functional data were coregistered and motion corrected. Voxels considered significantly activated were identified at ‘strong desire to void’ and ‘(attempt of) voiding initiation’ time points using a generalized linear model. Group level analysis was performed where statistically significant activation was considered if a voxel attained a P-value of less than 0.05. A t-test was utilized to compare activation of each ROI during initiation of voiding to the region’s baseline during the empty bladder phase. ROIs with a t-value greater than 2.1 were identified to be statistically significant.
RESULTS
Twenty participants (10 males and 10 females) signed the consent and completed the entire protocol as planned. MRI studies have suggested approximately 12–20 subjects to achieve 80% power on statistical analysis for typical activations [13,14]. Most published fMRI studies evaluating bladder function have included 8–12 subjects, for this reason we chose to include 10 participants per group. Table 1 shows participants’ demographics and results of voiding. Cohort-level BOLD analysis of “initiation of voiding” showed several statistically significant activation patterns in multiple regions. Within the brainstem specifically, 5 distinct ROIs were noted to have increased BOLD activation; 3 within the PAG, and 2 within the PMC (Fig. 3). These regions and their Montreal Neurological Institute coordinates are listed in Table 2 and shown in Fig. 4.
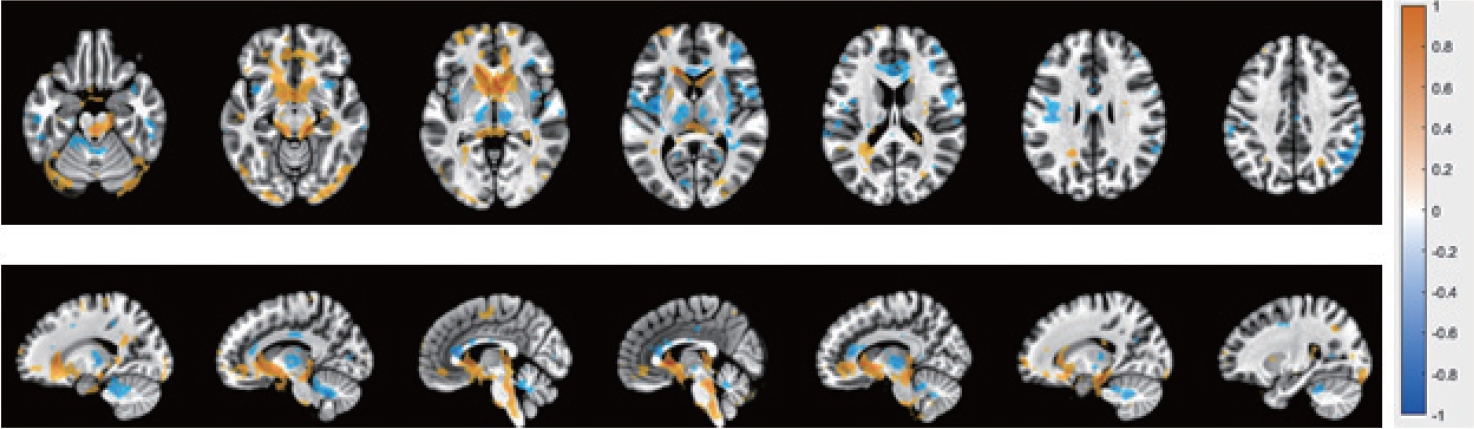
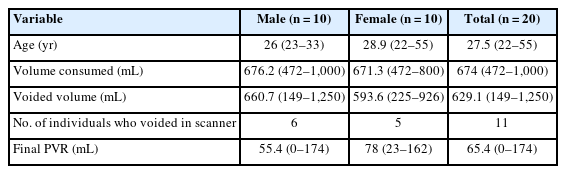
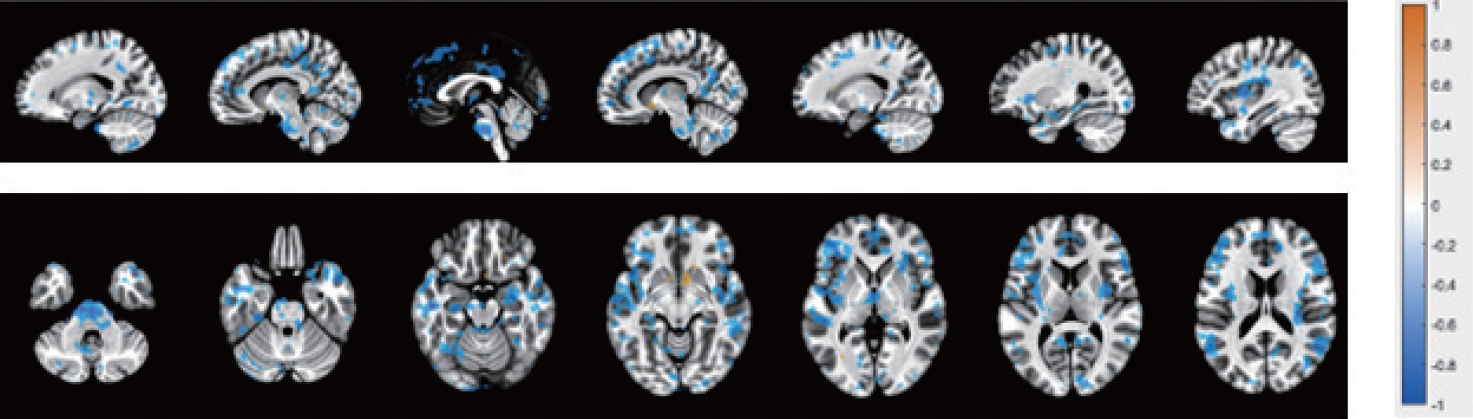
Blood oxygenation level dependent activation (orange) and inhibition (blue) in both genders during initiation of voiding.

Regions of interest with a significant blood oxygenation level dependent activation at “initiation of voiding” and their corresponding Montreal Neurological Institute coordinates
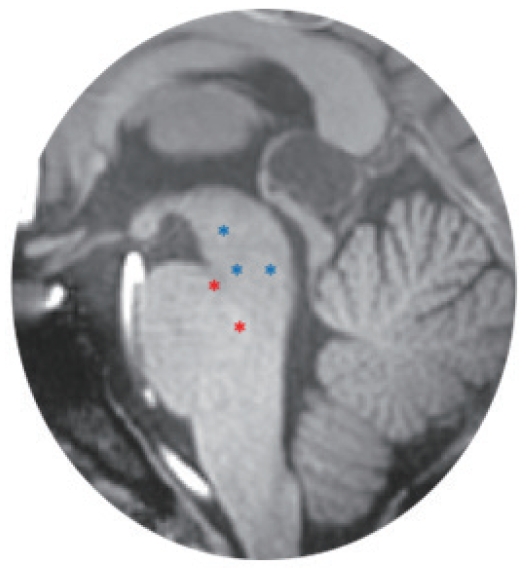
Sagittal magnetic resonance imaging of regions of interest within the brainstem with significant blood oxygenation level dependent activation at “initiation of voiding.” A red asterisk indicates an area of increased activation. A blue asterisk indicates an area of decreased activation (inhibition).
Further investigation into the difference between BOLD activation of each sex revealed a general decrease in BOLD activation for females compared to males, made apparent when performing a subtraction of the male activation signal from the overall cohort activation maps (Fig. 5). The only exception to this was the caudate, which demonstrated an increase in activation for females at the time of “initiation of voiding.”
DISCUSSION
This study investigated brain and specific brainstem activation patterns during the initiation of voiding in healthy males and females using ultra-high definition 7T fMRI. The results support the theory that although there are many similarities in the higher neural control of the bladder between male and female, there might be some differences. The inconsistencies in brain activation during voiding between males and females are not well researched. A metanalysis by Harvie et al. [15], assessed brain and brainstem regions involved in voiding in healthy adults in 9 studies and had similar overall findings as we present here. The investigators planned to compare the differences between healthy male and female volunteers, however, their sample size did not allow for that analysis [15]. This study is one of the first to investigate this difference between genders. While not fully understood, this could provide some insight into the higher occurrences of overactive bladder and incontinence in females [16-18]. During the initiation of voiding there is a widespread decrease in ROI activation in female participants compared to males with the exception of the caudate nucleus. This conclusion is consistent with results reported from Seseke et al. [19], where they found a stronger overall activation during contraction of pelvic floor muscles (resembling the initiation of voiding) in males compared to females and similar results during relaxation (end of voiding) as well as overall cortical activation of circuits involved in voiding between males and females. This may be explained by lower bladder capacities and more frequent voiding in females [20], as well as the inherent differences in the male and female genitourinary tract, such as the thicker detrusor muscles in males required to generate greater voiding pressure to push through the longer urethra. As a result, these anatomical variations may lead to the divergence in the activation patterns we observed in our reported results. Despite many studies on neural control of LUT ( > 20), few have evaluated actual voiding in men within the MRI scanner and have modest shortcomings. Blok et al. [3] used positron emission topography (PET) in 1997 and were the first to evaluate supraspinal regions involved in men voiding. This pivotal study was invasive and required intravenous administration of contrast, and brainstem localization integrity was limited due to PET technology, with reduced spatial definition and orientation. Later in 2015, Michels et al. [21] designed complex alternating randomized protocol blocks imitating micturition (furosemide as a diuretic) in men with 3T fMRI, and evaluated all supraspinal regions (brain and brainstem) activated during micturition.
As previously reported in literature, we saw BOLD activation in specific brainstem structures that have been proven to play a role in voiding, including the PAG and PMC [22]. In addition to the brainstem, other regions within the cerebrum demonstrate significant activation during initiation of voiding. Most notable of these regions are the left and right superior frontal gyrus, the left and right anterior cingulate gyrus, and the left and right parahippocampal gyrus. These 6 areas fit well into the current working model/circuits of LUT control in humans [6]. Several other studies have confirmed the importance of these regions in voiding control and specifically voiding initiation in humans [7]. In addition, regions associated with emotion, memory encoding, and retrieval were also observed to be activated during voiding initiation, including the caudate, hippocampus, and parahippocampal gyrus. Significant activation of the thalamus during initiation of voiding demonstrates that it is a major checkpoint role for all sensory information in the body. Other regions depicting significant activation are the caudate and putamen, 2 neural structures that comprise the basal ganglia. The basal ganglia assists in coordinating precise movements and modifying motor control. Proper interaction between the detrusor muscle, internal and external urinary sphincters, and the abdominal muscles are all necessary to ensure normal voiding, which could explain the basal ganglia’s role in coordinating this complex process.
As identified through analysis, there are many Talairach regions that show activation during voiding initiation. Several of these regions have already been shown to fit into the working model of brain-bladder circuits. However, there remains many more areas of the brainstem that also contribute to the process, either within these previously established circuits, or as yet to be discovered circuits. Additionally, our understanding of temporal activation pattern timing within and between these ROIs is scarce and should be considered in future investigations.
This study aimed to assess brain-bladder networks in young healthy individuals. Healthy young adults versus older individuals may demonstrate different activation patterns. Additionally, without using an invasive method of bladder filling, each subject’s rate of urine production and bladder filing as well as bladder size is variable. The unnatural setting of voiding while lying supine in an MRI machine might lead to a different pattern of brain activation compared to normal physiological settings. This contributes to some variance as when each subject feels the “desire to void” and whether they are capable of voiding for each of the 5 cycles. However, noninvasive bladder filling (passive hydration) is being utilized more frequently with improved participant tolerability and easier protocol reproducibility for LUT fMRI studies [23,24]. Furthermore, given the nature of our cycles to explore the initiation of voiding, investigating a more prolonged voiding phase may yield new information on what ROIs are incorporated in maintaining a proper flow of urine.
In conclusion, to our knowledge, this is the first study utilizing a noninvasive fMRI protocol with a high-resolution MRI scanner to investigate activation in both the brain and the brainstem during a brain-bladder protocol in healthy individuals (males and females), particularly during the initiation of voiding. The results support and confirm activation of specific regions within the PMC, PSC, and the PAG during voiding as well as the role they play in the initiation of voiding. For the first time, to our knowledge, a difference in activation has been shown in brain and brainstem neural activation patterns between healthy young males and females with an overall decrease in activation during voiding in the females. These findings may refine our understanding of current working brainbladder circuit models.
Notes
Grant/Fund Support
This study is partially supported by the NIH for this study (R03 DK126994).
Research Ethics
Eligible participants were screened and recruited following registration of our clinical trial (NCT04846387) and Institutional Review Board approval. Informed consent was obtained for each subject.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTION STATEMENT
· Conceptualization: BS, TB, RK
· Data curation: BS, DC, KT
· Formal analysis: BS, DC, KT, CK
· Funding acquisition: RK
· Methodology: BS, DC, KT
· Project administration: RK
· Visualization: BS, DC, KT, CK, BS
· Writing - original draft: BS
· Writing - review & editing: BS, DC, KT, BS, TB, RK
Acknowledgements
Authors would like to thank Ms. Hamida Rajab for her recruitment efforts and her regulatory support.