Kidney Transplantation and Clinical Outcomes in Patients With Multiple Myeloma: Evidence From the United States Nationwide Inpatient Sample
Article information
Abstract
Purpose
Patients with multiple myeloma (MM) are prone to developing persistent renal insufficiency. Novel therapeutic medications have improved long-term survival, making kidney transplantation (KT) a viable treatment option for MM survivors with end-stage renal disease. This study aimed to investigate the clinical outcomes in patients with MM who have received KT.
Methods
Data from hospitalized patients ≥ 40 years of age with MM in the Nationwide Inpatient Sample 2016–2018 of the United States were queried. Patients were classified as having or not having undergone KT, as well as the stage of chronic kidney disease (CKD) for those who had not received KT. Propensity-score matching (PSM) was applied to balance the characteristics between the groups. Binary logistic regression was utilized to determine the associations between study variables and inhospital mortality, unfavorable discharges, prolonged length of stay (LOS), and major complications.
Results
In total, 50,654 hospitalized patients with MM were identified, of whom 165 (0.3%) had received KT and 50,489 had not (5,905 at stage 5 CKD [CKD5D], 11,559 at stage 1–4 CKD [CKD1-4D], and 33,025 who were CKD-free). After PSM, between-group demographic and hospital-related characteristics were balanced. Binary regression analysis revealed that, compared to patients who were CKD-free, patients at CKD5D were significantly more likely to experience a prolonged LOS (odds ratio [OR], 1.31; 95% confidence interval [CI], 1.01–1.70) after adjusting for relevant confounders. Furthermore, compared to CKD-free patients, those who underwent KT were significantly more likely to have sepsis (OR, 1.48; 95% CI, 1.02–2.14). However, KT showed no association with the other adverse inpatient outcomes.
Conclusions
Although KT is not common in MM patients, those who had undergone KT had comparable hospital outcomes to CKD-free patients. These data will help clinicians deliver better consultations to MM patients attempting to receive KT.
INTRODUCTION
Multiple myeloma (MM) is a malignant tumor that originates in the bone marrow [1]. It is typically characterized by the neoplastic proliferation of plasma cells, which produce monoclonal immunoglobulins [2]. MM accounts for 2% of all cancers and approximately 10% of hematological malignancies, making it the second most common hematological malignancy. The disease’s most common clinical manifestations include bone damage, hypercalcemia, renal failure, and anemia [3]. The median age at diagnosis is 65 years, with a mere 3% of patients being under the age of 40 at presentation [4]. As MM progresses, the normal balance between osteoblast and osteoclast activity shifts, favoring net bone loss. Most cases of MM are associated with an initial somatic mutation, often accompanied by additional oncogenic mutations. The complex genetic makeup of the disease makes treatment for MM difficult [4].
Patients with high-risk MM typically survive less than 5 years, with a significant portion of deaths occurring within the first 2 years postdiagnosis [4]. Acute kidney injury (AKI) is a condition that largely mirrors the extent of the tumor burden [5]. Over the course of their disease, at least one AKI episode will be experienced by half of the patients with MM. Approximately 50% of these patients will have reversible renal insufficiency, but the rest will exhibit some level of persistent chronic kidney disease (CKD), with 2%–12% necessitating renal replacement therapy [6]. The most prevalent cause of persistent renal insufficiency in MM is tubular nephropathy, which results from monoclonal immunoglobulins secreted by plasma cell clones [7]. Clinically, the primary form of kidney injury in MM is light chain cast nephropathy [8].
AKI and progressive CKD typically result in end-stage renal disease (ESRD), which is linked to a poor prognosis in MM [9]. The median survival rate for MM patients who develop ESRD is a mere 18.3 months [10]. In the past, MM was deemed incurable, but the introduction of innovative therapeutic drugs has enhanced the survival rates of MM patients. Treatments such as anti-myeloma induction therapy, subsequent autologous stem cell transplantation, and maintenance therapy all contribute to extending the period of disease-free remission [11]. Until recently, kidney transplantation (KT) was not viewed as a feasible treatment option for MM survivors with ESRD [12]. However, there is a growing body of evidence suggesting that some MM patients can successfully undergo KT following autologous stem cell transplantation [13].
However, it is generally believed that individuals with MM who have developed ESRD tend to fare poorly after undergoing KT, often due to increased mortality rates from the cancer itself or related comorbidities [14]. While kidney function can be normalized in kidney transplant recipients (KTRs), these individuals require additional care due to their long-term use of immunosuppressants [15]. There is a notable lack of studies evaluating the performance of MM patients post-KT, particularly on a population-wide scale. Therefore, this study aims to address this knowledge gap by investigating the clinical outcomes of MM patients who have previously undergone a KT, using a large, nationally representative database.
MATERIALS AND METHODS
Study Design and Data Source
The data used in this population-based, retrospective analysis was sourced from the United States (US) Nationwide Inpatient Sample (NIS) database. This database is the largest of its kind, encompassing all-payer inpatient care in the US, and it records approximately 8 million hospital stays annually [16]. The National Institutes of Health’s Healthcare Cost and Utilization Project (HCUP) manages the NIS database. It includes a comprehensive range of information, such as diagnoses, procedures, discharge status, patient demographics, payment source, and hospital-associated characteristics. The NIS database is regularly updated and gathers data from approximately 1,050 hospitals across 44 US states, which represents a 20% sample of US community hospitals.
Ethics Statement
This study complies with the NIS data-use agreement. Data were obtained by submitting a request to the administrator of the database, the Online HCUP Central Distributor (certificate# HCUP-1J23CWS19). As we utilized precollected data from the NIS database, patients were not directly involved. The study protocol was presented to the Institutional Review Board (IRB) of Chinese PLA General Hospital, which granted an exemption from IRB approval. The need for informed consent was also waived, as all data is deidentified.
Selection of Study Population
In this study, we utilized the International Classification of Diseases, 10th Edition (ICD-10) diagnosis codes to identify individuals aged 40 and above, diagnosed with MM (ICD-10: C90.0), who were admitted to US hospitals between 2016 and 2018. We excluded patients with other types of malignancies, those with a history of KT graft failure, and those with missing data on sex, CKD stage, or relevant outcomes. The study population was then divided into 2 categories: KTRs (ICD-10: Z94.0), and patients who had not undergone a prior KT (grouped as stage 5 CKD [CKD5D], stage 1–4 CKD [CKD1-4D], and those free of CKD). To differentiate patients with CKD stages 1–5, we used ICD-10 codes N18.1-N18.6.
Variables and Outcome of the Study
The primary outcomes of the study included: (1) in-hospital mortality; (2) extended length of stay (LOS); (3) unfavorable discharge, defined as being transferred to nursing homes or long-term care facilities; and (4) major complications. These complications encompassed cerebrovascular accident, acute myocardial infarction, respiratory failure, pneumonia, sepsis, severe infection, deep vein thrombosis/pulmonary embolism, and mechanical ventilation. An extended LOS was defined as a hospital stay exceeding the 75th percentile (i.e., 8 days) of the study cohort. Each major complication was identified using the ICD-10 code system.
Covariates
The characteristics of the patients included their age, sex, ethnicity, income, and insurance status (primary payer). The Charlson Comorbidity Index (CCI), which was identified through ICD codes, was utilized to represent the overall severity of the patients’ comorbid conditions. Hospital-related variables such as bed size, location/teaching status, and hospital region were obtained from the database and made available to all patients as part of the full data set.
Statistical Analysis
We utilized the SAS functions PROC SURVEYFREQ and PROC SURVEYMEANS to calculate descriptive statistics and group comparisons. Categorical data are represented by unweighted numbers (n) and weighted percentages (%), while continuous data are described by the mean ± standard error. Patients in the case group (KTR) and various control groups (those free of CKD [CKD-free], patients with CKD1-4D, and patients with CKD5D) were matched using the propensity-score matching (PSM) method. The case-to-control ratio was 1:4, based on factors such as age category, race, sex, income, primary payer, hematopoietic stem cell transplantation (HSCT), CCI category, location/teaching status, hospital region, and hospital bed number. We then employed logistic regression with the PROC SURVEYLOGISTIC statement to calculate odds ratios (ORs) and 95% confidence intervals (CIs) for the relationships between study variables and outcomes. To ensure the representation of nationwide studies, we created weighted samples by combining stratum (NIS_STRATUM), cluster (HOSPID), and weight values (TRENDWT before 2011 and DISCWT after 2012). We defined statistical significance as a two-sided P-value of < 0.05. All statistical analyses were conducted using SAS ver. 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Study Population Selection
Fig. 1 illustrates the process of selecting the study cohort. In this study, we identified data from 67,688 hospitalized patients aged 40 years or older with MM, as recorded in the NIS database. After excluding patients with missing information on key variables (n = 17,034), 50,654 individuals remained as the study population (Fig. 1).
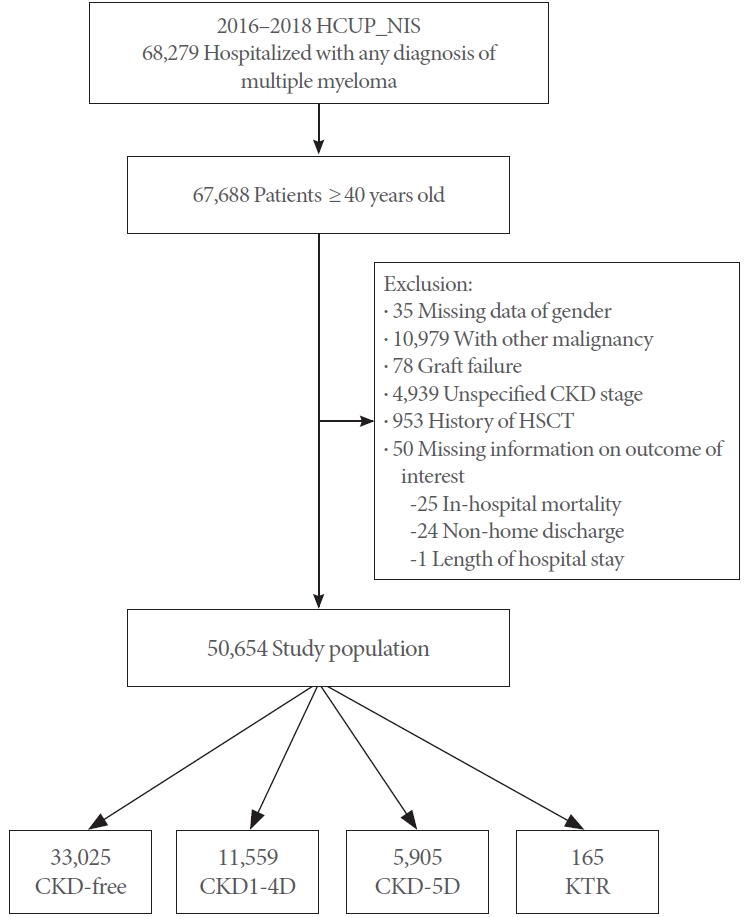
Flow diagram of the study cohort selection. HCUP_NIS, Healthcare Cost and Utilization Project_Nationwide Inpatient Sample; CKD, chronic kidney disease; HSCT, hematopoietic stem cell transplantation; CKD1-4D, stage 1-4 CKD; CKD5D, stage 5 CKD; HMO, health maintenance organization; KTR, kidney transplant recipient.
Characteristics of Hospitalized Patients With MM
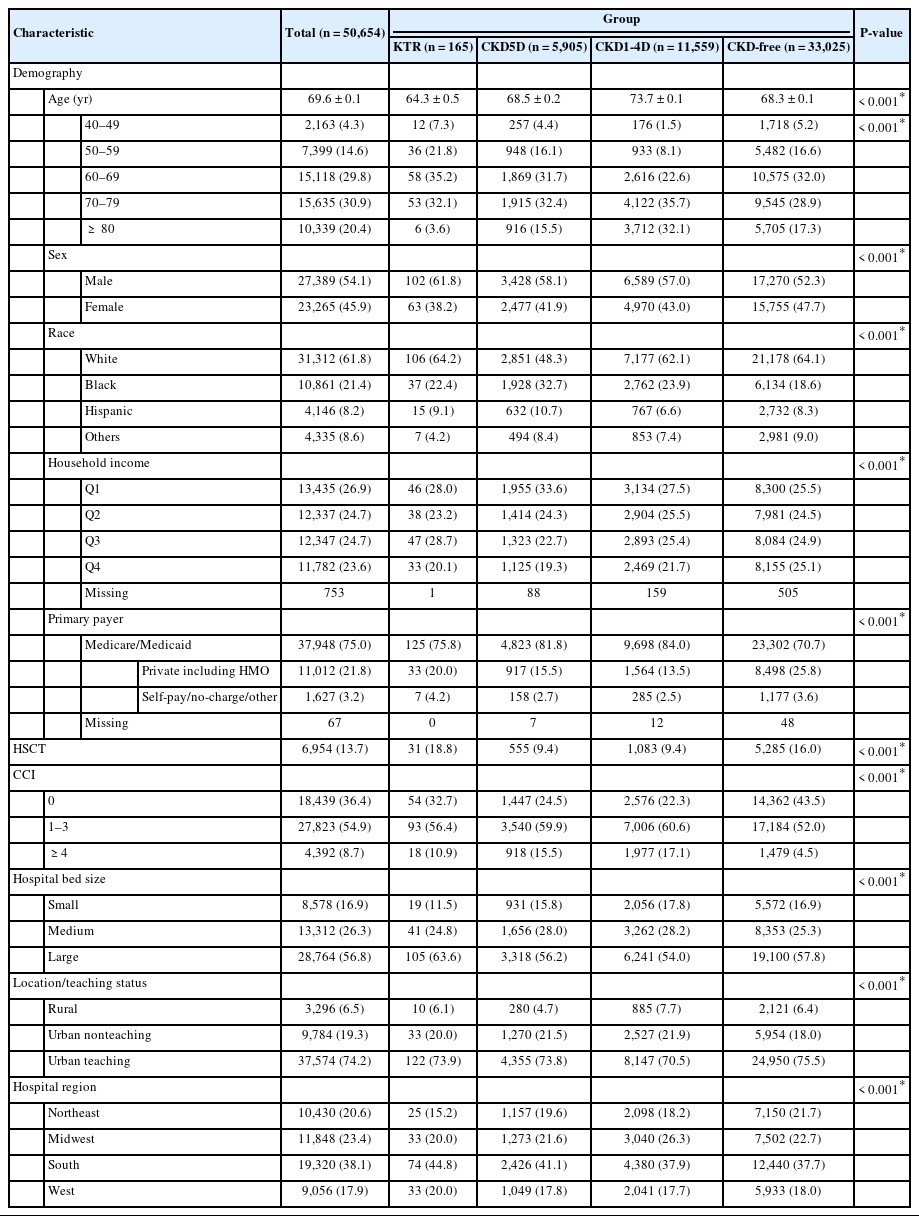
Patient- and hospital-level characteristics before PSM are summarized in Table 1. The mean age of the study population was 69.6 years. Of this population, 27,389 (54.1%) were male and 31,312 (61.8%) were White. A total of 165 patients had undergone KT, while 50,489 had not. Among those who had not received KT, 5,905 were at CKD5D, 11,559 were at CKD1-4D, and 33,025 were CKD-free. Specifically, KTRs were younger and had higher percentages of males, Whites, those with moderate household income, and those with insurance covered by self-pay/no-charge/other. KTRs also had a higher percentage of history of HSCT than those who were CKD-free, at CKD1-4D, and at CKD5D (18.8% vs. 16.0%, 9.4%, and 9.4%, respectively, P < 0.001).
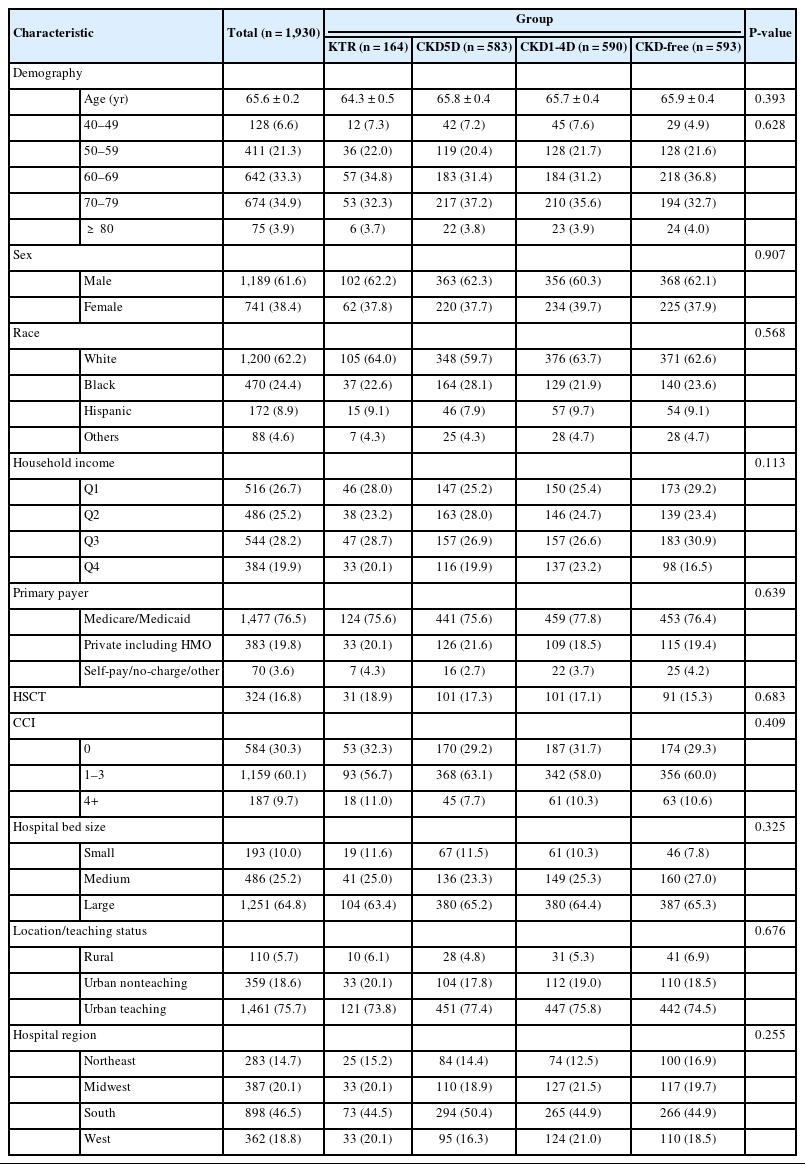
The characteristics of the study population following PSM are detailed in Table 2. There were no significant differences in the distribution of all demographic and hospital-related characteristics across the 4 groups (Table 2).
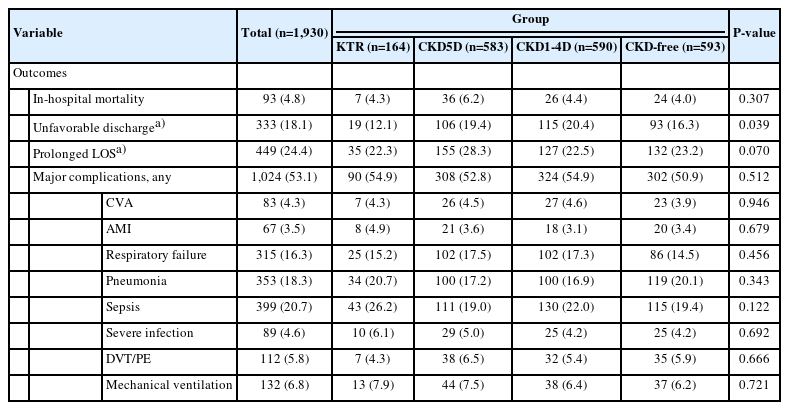
Clinical Outcomes of Patients With MM by CKD Status After PSM
The clinical outcomes of patients with MM are summarized in Table 3. No significant differences in in-hospital mortality, prolonged LOS, or major complications were observed among the 4 groups. Patients with KT had the lowest percentages of unfavorable discharges compared to the other groups (12.1% and 16.3% vs. 19.4% and 20.4%, respectively, P = 0.039) (Table 3).
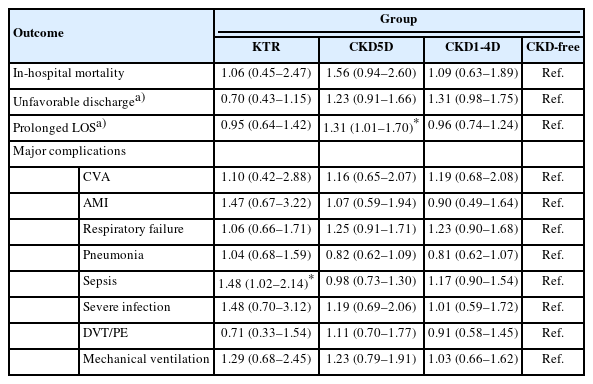
Associations Between Clinical Outcomes and Status of KT and CKD
The associations between clinical outcomes and patients’ KT and CKD status are summarized in Table 4. Binary regression analysis showed that, compared to those without CKD, patients with CKD5D had significantly higher odds of experiencing a prolonged LOS (OR, 1.31; 95% CI, 1.01–1.70) if they also had MM. Furthermore, KTRs were significantly more likely to experience sepsis (OR, 1.48; 95% CI, 1.02–2.14) than patients without CKD (Table 4).
DISCUSSION
To the best of our knowledge, this is the first population-based study to compare the outcomes of patients with MM who underwent KT to patients without KT at different CKD statuses. In the present study, we found that, as compared to CKD-free patients, MM patients with CKD5D were prone to having prolonged LOS. KTRs generally had no excessive risk regarding inhospital mortality, unfavorable discharge, or major complication over CKD-free individuals, except for an approximately 50% excess risk of sepsis.
A previous study reported that despite advances in the treatment of MM, CKD remains a common complication. Renal impairment is a common condition in patients with MM, with approximately 25% of patients presenting with renal insufficiency and approximately 50% developing renal disease over the course of the disease [17]. Although ESRD leads to a poorer prognosis in patients with MM, survival has improved [17]. Recent research suggests that certain patients with MM can successfully undergo KT, though immunomodulating drugs should be used with caution [18].
Patients with MM may exhibit various forms of kidney injury, such as cast nephropathy and monoclonal immunoglobulin renal deposition disease. Cast nephropathy is the most frequently observed pattern in myeloma patients with renal involvement [19]. This condition is primarily characterized by the formation of clear cylinders and proximal tubular damage, which are caused by free light chains in the urine [20]. There are also other subsets of patients with kidney involvement who develop monoclonal immunoglobulin deposition disease [21].
Over half of patients with cast nephropathy failed to regain their kidney function, resulting in severe CKD, including ESRD [22]. Prior research suggested that KT could be a feasible treatment option for MM, although this was based on limited data [23,24]. For MM patients with ESRD who are considering KT, induction therapy against myeloma, followed by autologous stem cell transplantation and maintenance therapy, is suitable for both standard-risk and high-risk patients [24].
This study found that KTRs were at a significantly higher risk of sepsis compared to individuals without CKD, but not at a higher risk of pneumonia. Furthermore, the major complications in patients with CKD5D on dialysis did not significantly differ from those in CKD-free patients. The literature has extensively documented that due to lifelong immunosuppression, solid organ transplant recipients, such as KTRs, are at a heightened risk of infectious complications [25]. However, our analysis did not show a significant increase in pneumonia risk among KTRs. KTRs are often under close medical supervision, and preventive measures may be implemented to lower the risk of certain infections, including pneumonia. For instance, KTRs might receive vaccinations against specific bacterial pathogens, which could help reduce the risk of pneumonia to a certain degree. Similarly, the vigilant and proactive medical care provided to CKD5D patients could lead to early detection and prompt treatment of any health issues, potentially decreasing the risk of severe complications during hospitalization.
Our study did not assess the relapse rate following KT in patients with MM. A case series by Shah et al. revealed that within 2 years post-KT, 40% of their cohort experienced a relapse of MM. Moreover, both graft survival and patient survival rates were 80% at the 4-year mark [26]. A separate recent study found that KT outcomes were more favorable for patients with myeloma who had received bortezomib either before or after the KT. Despite these findings, relapse post-KT in MM patients remains a common occurrence. Therefore, future research is needed to identify the patient subgroups that would most benefit from KT [27].
Taken together, our study and the previous case series highlight the utility and outcomes of KT in patients with MM. Future longitudinal studies are needed to provide updated evidence regarding the impact of KT on outcomes of MM.
The primary strength of this study lies in its utilization of large-scale national representative data, providing insight into real-world circumstances regarding how inpatient outcomes among patients with MM correlate with their underlying kidney function statuses. However, the interpretive scope of the study is limited by its retrospective and observational design. Potential coding errors could impact the reliability of the analyses. The study also lacks laboratory parameter data and long-term follow-up data post-discharge. The exact disease state of MM and the precise kidney function of the patients were not known, factors that could significantly affect the observed outcomes. Moreover, due to the varied reasons for patients’ hospital admissions, it is essential to interpret the findings with caution. Nonetheless, it is important to acknowledge the study’s efforts to minimize bias by meticulously matching patients based on several key baseline characteristics, including age, race, sex, comorbidities, and other pertinent demographic and hospital-related characteristics. Consequently, it is reasonable to infer that the distribution of admission causes was balanced among the groups, thereby emphasizing kidney function status as the primary factor potentially influencing in-hospital outcomes.
In conclusion, although KT is not common among MM patients, those who had received KT had comparable hospital outcomes to CKD-free patients. These insights could assist physicians in providing more effective consultations to MM patients considering KT.
Notes
Grant/Fund Support
The study was supported by the New Technology and Business Supporting Project of Chinese PLA General Hospital (XYW-202107), Military Healthcare Special Project (19BJZ28).
Research Ethics
The study protocol was presented to the Institutional Review Board (IRB) of Chinese PLA General Hospital, which granted an exemption from IRB approval. The need for informed consent was also waived, as all data is deidentified.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTION STATEMENT
· Conceptualization: BY, LZ, XL
· Data curation: BY, LZ, XL
· Formal analysis: BY, LZ, XL
· Funding acquisition: XL
· Methodology: BY, LZ
· Project administration: BY
· Writing - original draft: XL
· Writing - review & editing: BY, XL