Adenosine A2A Receptor Agonist, Polydeoxyribonucleotide Treatment Improves Locomotor Function and Thermal Hyperalgesia Following Neuropathic Pain in Rats
Article information
Abstract
Purpose
Lithotomy position has been widely used in the various urologic surgery. Occasionally sensory and motor problems of the lower extremities are occurred due to the lithotomy position and these deficits may be related with sciatic nerve injury (SNI). Inflammatory process is a factor to induce functional impairment after SNI. Therefore, we evaluated the role of adenosine A2A receptor agonists, polydeoxyribonucleotide (PDRN) showing anti-inflammatory effect on locomotor function following SNI in rats.
Methods
Sciatic nerve was compressed with surgical clips for 1 minute after exposing of right sciatic nerve. After 3 days of SNI, PDRN (2, 4, and 8 mg/kg) was applied to the damaged area of sciatic nerve once daily for 10 days. Walking track analysis was conducted for locomotor function and plantar test was performed for thermal pain sensitivity. Level of cyclic adenosine-3´,5´-monophosphate (cAMP) were measured using enzyme-linked immunosorbent assay. Western blot analysis was performed for tumor necrosis factor (TNF)-α, interleukin (IL)-1β, cAMP response element binding protein (CREP), vascular endothelial growth factor (VEGF). Immunofluorescence for neurofilament was also conducted.
Results
Locomotor function was decreased and thermal pain sensitivity was increased by SNI. SNI enhanced proinflammatory cytokines’ production, such as TNF-α and IL-1β, while suppressed CREP phosphorylation and cAMP level. SNI also reduced the expression of VEGF and neurofilaments. However, treatment with PDRN inhibited proinflammatory cytokines’ production and upregulated CREP phosphorylation and cAMP expression. PDRN also enhanced the expression of VEGF and neurofilaments. As a result, PDRN improved locomotor function and alleviated thermal hyperalgesia after SNI.
Conclusions
PDRN has shown potential to be used as an effective treatment for neuropathic pain.
• HIGHLIGHTS
- Locomotor function was decreased and thermal pain sensitivity was increased by sciatic nerve injury (SNI).
- Polydeoxyribonucleotide (PDRN) improved locomotor function and alleviated thermal hyperalgesia after SNI.
- PDRN has shown potential to be used as an effective treatment for neuropathic pain.
INTRODUCTION
Peripheral nerve injury can cause functional loss and reduce quality of life due to secondary problems such as permanent damage to sensory/motor functions and neuropathic pain [1,2]. Lithotomy position has been widely used in the various urologic surgery. Occasionally sensory and motor problems of the lower extremities are occurred due to the lithotomy position and these deficits may be related with peripheral nerve injury such as sciatic nerve injury (SNI) [3].
Among various peripheral nerve injuries, SNI is one of the neuropathies that causes painful symptoms. The affected limbs exhibit abnormal gait, hyperalgesia, and edema, and these symptoms worsen due to acceleration of inflammatory activity [4,5]. Damage to peripheral nerves releases various substances involved in nociceptive signaling, such as proinflammatory cytokines, in the early stages [6].
In particular, peripheral nerve injury in the acute phase enhances the secretion of many proinflammatory cytokines, including tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-2, IL-6, and IL-8. On the other hand, anti-inflammatory cytokine expression was suppressed by inducing peripheral nerve injury [7,8]. Ultimately, the level of inflammation in the peripheral nerves is linked to the severity of pain. Various drugs have been applied to treat SNI, but side effects and low efficacy have been problems. Therefore, an anti-inflammatory agent that has no side effects and is highly effective in the early stages is needed.
One of adenosine A2A receptor agonists, polydeoxyribonucleotide (PDRN), is made from salmon sperm and is known to exert anti-inflammatory effect through suppressing proinflammatory cytokines’ production [9]. A2A receptor stimulation by PDRN reduces proinflammatory cytokines’ production and results in inhibition of apoptosis in various disease conditions [10,11]. Furthermore, PDRN promotes wound healing by stimulating vascular endothelial growth factor (VEGF) expression through interaction with adenosine A2A receptor [12].
PDRN accelerates healing process and suppressed inflammatory event, but the exact action of PDRN on SNI is not clear. In the current study, we studied the PDRN’s effect on neuropathic pain caused by sciatic nerve compression, focusing on the relation of cyclic adenosine-3´, 5´-monophosphate (cAMP) level. We evaluated the therapeutic efficacy of PDRN on locomotor function, thermal hyperalgesia, and inflammatory state after SNI in rats.
MATERIALS AND METHODS
Animals and Classification
Sprague-Dawley male rats (230±10 g, 10 weeks in age) were obtained from the Orient Bio Company (Seongnam, Korea). Rats were raised in a temperature (23°C±2°C) and lighting (08:00 to 20:00 hours) controlled laboratory and had access to food and water at libitum. Rats were randomly classified as the 5 groups (n=10 in each group): sham operating group, SNI inducing group, SNI inducing with 2-mg/kg PDRN treating group, SNI inducing with 4-mg/kg PDRN treating group, SNI inducing with 8-mg/kg PDRN treating group. This experimental procedure was conducted after approval from the Kyung Hee University’s ethics committee and the approval number was KHUASP(SE)-21-348.
SNI Induction
Surgical procedure was applied to cause crush injury in the SNI inducing groups [5,13]. Incising on the gluteal muscle was made following anesthesia by intraperitoneal application of Zoletil 50 (10 mg/kg; Vibac Laboratories, Carros, France), and then exposed right sciatic nerve. Surgical clips (pressure: 125 g; Fine Science Tool Inc., San Francisco, CA, USA) was applied to crush the sciatic nerve for 1 minute.
Drug Application
After 3 days of SNI, PDRN (Rejuvenex, PharmaResearch Products, Pangyo, Korea) was injected into the damaged area of sciatic nerve once a day at each dose (200 μL) for 10 days to the PDRN treating groups. During the same period, 200 μL 0.9% NaCl was injected in0to the right sciatic nerve in the sham operating group and in the SNI inducing group.
Walking Tract Analysis
Sciatic functional index (SFI) is well known indicator showing functional recovery following nerve injury. On days 0, 4, 7, and 10 after SNI, walking track analysis was done as the following explained method [14]. Rat tracks were recorded on wooden passages (8.2×42 cm) whose floor was covered with white paper. Print length (PL) represents the distance from the heel to the top of the 3rd toe. Toe spread (TS) represents the distance between the 1st to the 5th toe. Intermediary toe spread (IT) represents the distance from the 2nd to the 4th toe. These items were measured from the footprint, and these items were calculated from the uninjured left foot (nonsurgical; NPL, NTS, NIT) and the injured right foot (experimental; EPL, ETS, EIT). Print length factor (PLF) was calculated from the (EPL-NPL)/NPL, toe spread factor (TSF) was calculated from the (ETS-NTS)/NTS, and intermediary toe spread factor (ITF) was calculated from the (EIT-NIT)/NIT. The SFI value was obtained using following formula SFI=-38.3×PLF+109.5×TSF+13.3×ITF-8.8. The SFI value close to 0 indicates normal rat, while the SFI value close to -100 represents complete disability.
Plantar Test
Thermal hyperalgesia detected by plantar test [15]. Rats were placed in a plastic box, allowed to acclimate for 5 minutes, and then subjected to thermal hyperalgesia test using a footpad test algesimeter (Ugo-Basile, Comerio, Italy). When radiant heat was applied to the plantar surface of the rat’s hind paw, the time until the rat avoided the paw was measured.
Tissue Preparation
Tissue preparation was carried out as described below [16]. Ten days after SNI induction, rats were sacrificed by Zoletil 50 (50 mg/kg, intraperitoneal; Vibac Laboratories) anesthesia. After extraction of sciatic nerve tissues, sciatic nerve tissues were fixed by 4% paraformaldehyde, and dehydrated in 70%, 80%, 90%, 95%, and 100% ethanol. Sciatic nerve tissues were incubated with xylene, and then embedded in paraffin. Paraffinembedded tissues were cut into 5-μm-thick sections by a microtome (Thermo Fisher Scientific, Waltham, MA, USA), and the tissues were mounted on coated slides. Slides containing tissues were dried on a hot plate overnight at 37°C. Some tissues for western blot and enzyme-linked immunoassay (ELISA) analysis were immediately frozen at -80°C.
cAMP Concentration Measurement
Using a cAMP enzyme immunoassay kit (Abcam, Cambridge, UK), cAMP concentration in sciatic nerve tissues was calculated by ELISA method [17] as described by manufacturer’s instructions.
Western Blotting
Western blotting for TNF-α, IL-1β, VEGF, and cAMP response element binding protein (CREP), was performed according to methods described previously [18]. Sciatic nerve tissues were homogenized in lysis buffer. Following centrifugation for 30 minutes at 14,000 rpm, proteins were detected. Table 1 shows the primary and secondary antibodies used in this experiment.
Immunofluorescence
Immunofluorescence for neurofilament was performed as follows [19]. Using xylene and graded ethanol, paraffin slides containing sciatic nerve tissues were deparaffinized for 5 minutes, then washed in distilled water for 5 minutes. Next, the slides were placed in 10mM sodium citrate buffer and boiled at 95°C for 2 minutes. After cooling at room temperature for 30 minutes, the slides were blocked with phosphate-buffered saline with 5% normal goat serum (Vector Laboratories, Burlingame, CA, USA) and 0.3% triton X-100. Next, tissue slides were treated with primary antibodies on neurofilament proteins (Sigma Chemical Co., St. Louis, MO, USA) at 4°C overnight. Secondary fluorescein isothiocyanate anti-mouse IgG antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) were applied to the slides at room temperature after washing. The slides were mounted on coverslips using Vectashield with 4´,6-diamidino-2-phenylindol (Vector Laboratories). Images were taken using a Leica DMi8 fluorescence microscope (Leica Microsystems, Wetzlar, Germany) at wavelengths of excitation 490 nm and emission 525 nm for Alexa 488.
Data Analysis
For the multiple comparisons, IBM SPSS Statistics ver. 22.0 (IBM Co., Armonk, NY, USA) was conducted. Statistical analysis was done by 1-way analysis of variance and Duncan post hoc test. The data were presented as mean±standard error of the mean. In the results, P<0.05 indicated statistical significance.
REULSTS
Locomotor Function Change
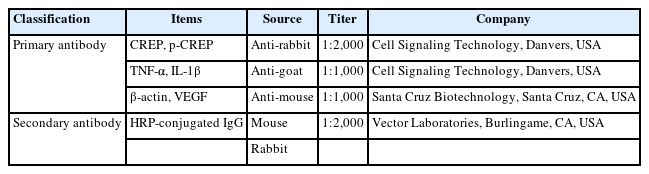
PDRN’s effect on SFI, showing functional recovery, after SNI is shown in Fig. 1. After SNI induction, SFI was measured on days 5, 7, and 9. As presented in Fig. 1, the SFI value in the sham-operated group was close to 0, indicating that the sciatic nerve had normal function. In the SNI group, SFI dropped to close to -100, and SFI gradually increased over the course of the experiment. In the PDRN treatment groups, on the other hand, an increment of SFI was observed 7 days after SNI induction (P<0.05). At day 9 after SNI induction, accelerated recovery was more evident in the 8-mg/kg PDRN treatment group (P<0.05). The current data demonstrated that treatment with PDRN promotes improvements in locomotor function following SNI induction.
Effect of PDRN treatment on sciatic functional index (SFI) following SNI. (A) Walking track foot prints in SNI. (B) Plot showing the time-dependent changes in SFI value in each group. (○) Sham operating group, ( ) SNI inducing group, (◊) SNI inducing with 2-mg/kg PDRN treating group, (∆) SNI inducing with 4-mg/kg PDRN treating group, (□) SNI inducing with 8-mg/kg PDRN treating group. PDRN, polydeoxyribonucleotide; SNI, sciatic nerve crush injury; NIT, normal intermediate toe spread; NPL, normal total print length; NTS, normal toe spread; EIT, experimental intermediary toe spread; EPL, experimental print length; ETS, experimental toe spread. P<0.05 compared to the sham operating group. #P<0.05 compared to the SNI inducing group.
) SNI inducing group, (◊) SNI inducing with 2-mg/kg PDRN treating group, (∆) SNI inducing with 4-mg/kg PDRN treating group, (□) SNI inducing with 8-mg/kg PDRN treating group. PDRN, polydeoxyribonucleotide; SNI, sciatic nerve crush injury; NIT, normal intermediate toe spread; NPL, normal total print length; NTS, normal toe spread; EIT, experimental intermediary toe spread; EPL, experimental print length; ETS, experimental toe spread. P<0.05 compared to the sham operating group. #P<0.05 compared to the SNI inducing group.
Thermal Pain Sensitivity Change
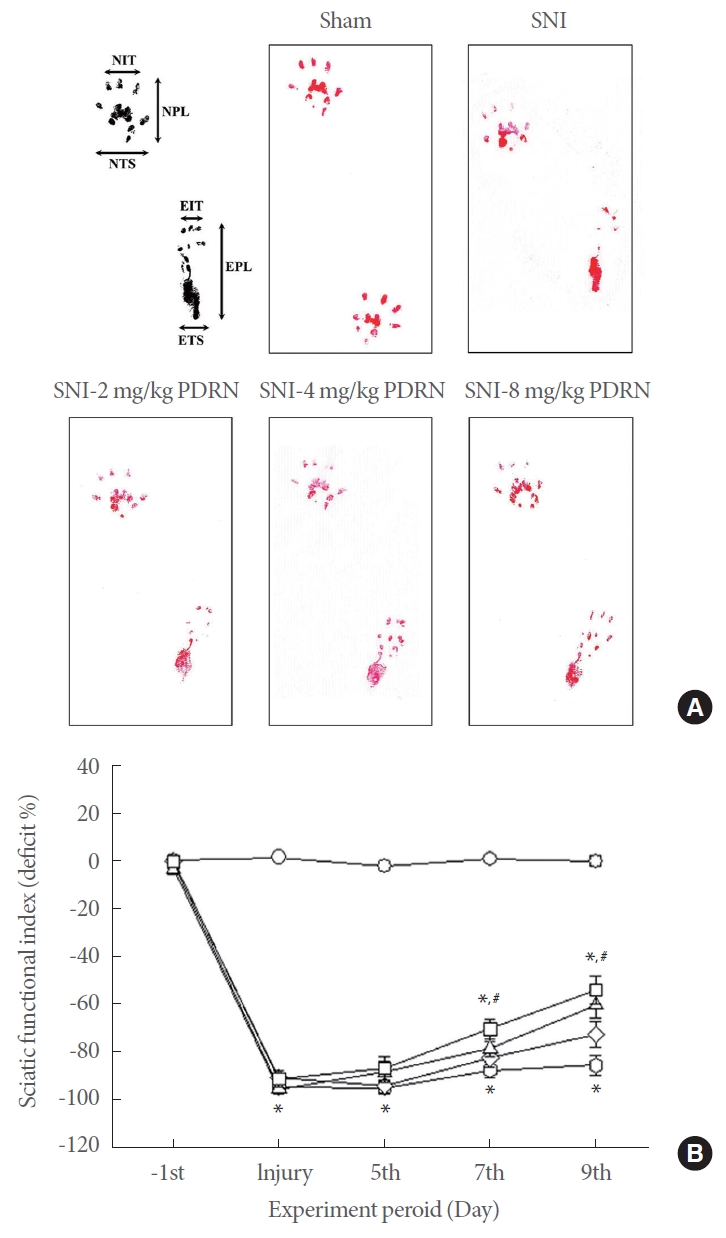
Thermal pain sensitivity was detected by plantar test and expressed as paw withdrawal latency (Fig. 2). Inducing SNI decreased paw withdrawal latency (P<0.05), whereas treating with PDRN lengthened paw withdrawal latency (P<0.05). The current data demonstrated treatment with PDRN alleviated SNI-induced pain, and that high-dose PDRN treatment, in particular, was most effective in relieving neuropathic pain.
Effect of PDRN treatment on paw withdrawal latency in plantar test. Sham, Sham operating group; SNI, SNI inducing group; SNI-2P, SNI inducing with 2-mg/kg PDRN treating group; SNI-4P, SNI inducing with 4-mg/kg PDRN treating group; SNI-8P, SNI inducing with 8-mg/kg PDRN treating group. PDRN, polydeoxyribonucleotide; SNI, sciatic nerve crush injury. *P<0.05 compared to the sham operating group. #P<0.05 compared to the SNI inducing group.
Pro-inflammation Cytokines Expression
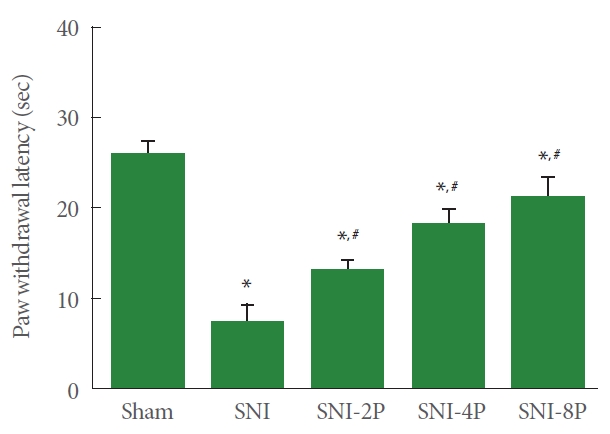
The expression of TNF-α and IL-1β, proinflammatory cytokines, in sciatic tissue is presented in Fig. 3. Induction of SNI enhanced TNF-α and IL-1β expression in sciatic nerve tissues (P<0.05). However, PDRN treatment suppressed TNF-α and IL-1β expression. Treating with 8 mg/kg PDRN suppressed expression of TNF-α and IL-1β most potently than low-dose PDRN treatment (P<0.05).
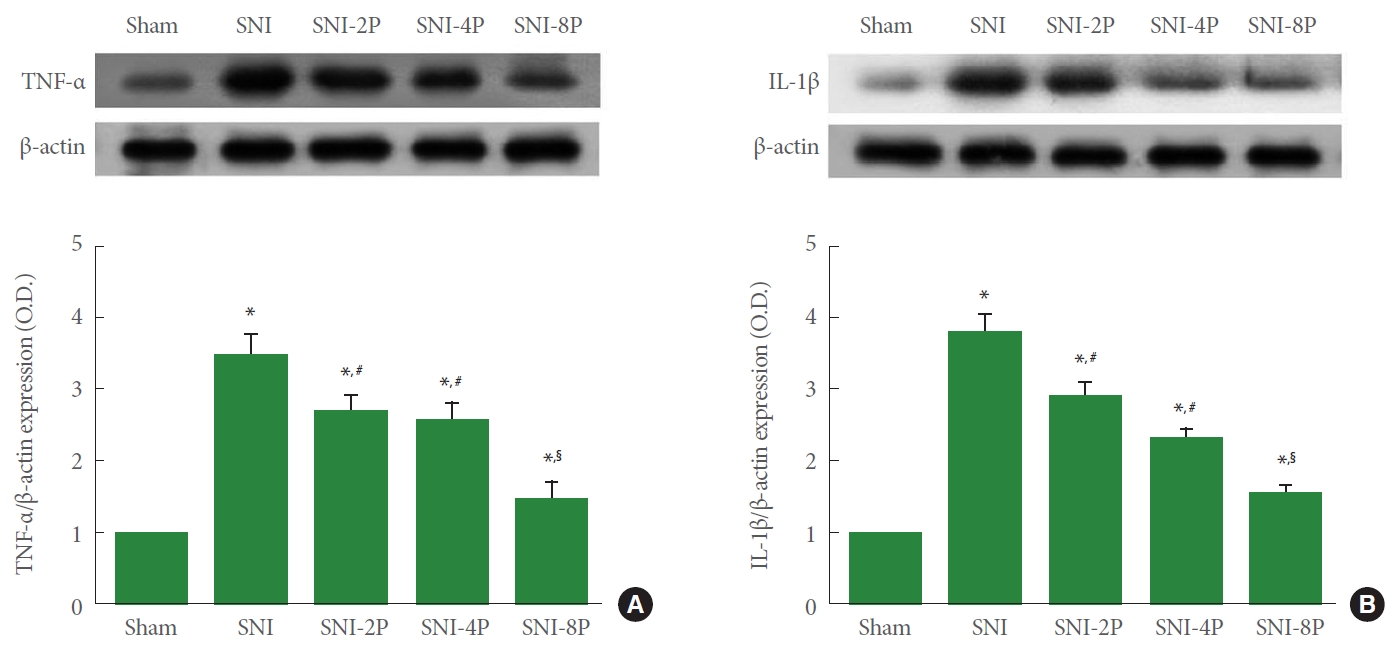
Effect of PDRN treatment on the expression of proinflammatory cytokines in sciatic nerve tissues. (A) TNF-α expression. Upper panel: representative expression of TNF-α. Lower panel: relative expression of TNF-α. (B) IL-1β expression. Upper panel: representative expression of IL-1β. Lower panel: relative expression of IL-1β. Sham, Sham operating group; SNI, SNI inducing group; SNI2P, SNI inducing with 2-mg/kg PDRN treating group; SNI-4P, SNI inducing with 4-mg/kg PDRN treating group; SNI-8P, SNI inducing with 8-mg/kg PDRN treating group. PDRN, polydeoxyribonucleotide; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; SNI, sciatic nerve crush injury. *P<0.05 compared to the sham operating group. #P<0.05 compared to the SNI inducing group. §represents P<0.05 compared to the 2 mg/kg PDRN treating group.
CREP Phosphorylation and cAMP Expression
Western blot analysis identified the effect of PDRN on phosphorylation of CREP in sciatic tissue (Fig. 4). Induction of SNI suppressed CREP phosphorylation (P<0.05), whereas treating with PDRN upregulated CREP phosphorylation in sciatic nerve tissues. In particular, 8 mg/kg PDRN treatment most strongly phosphorylated CREP (P<0.05).
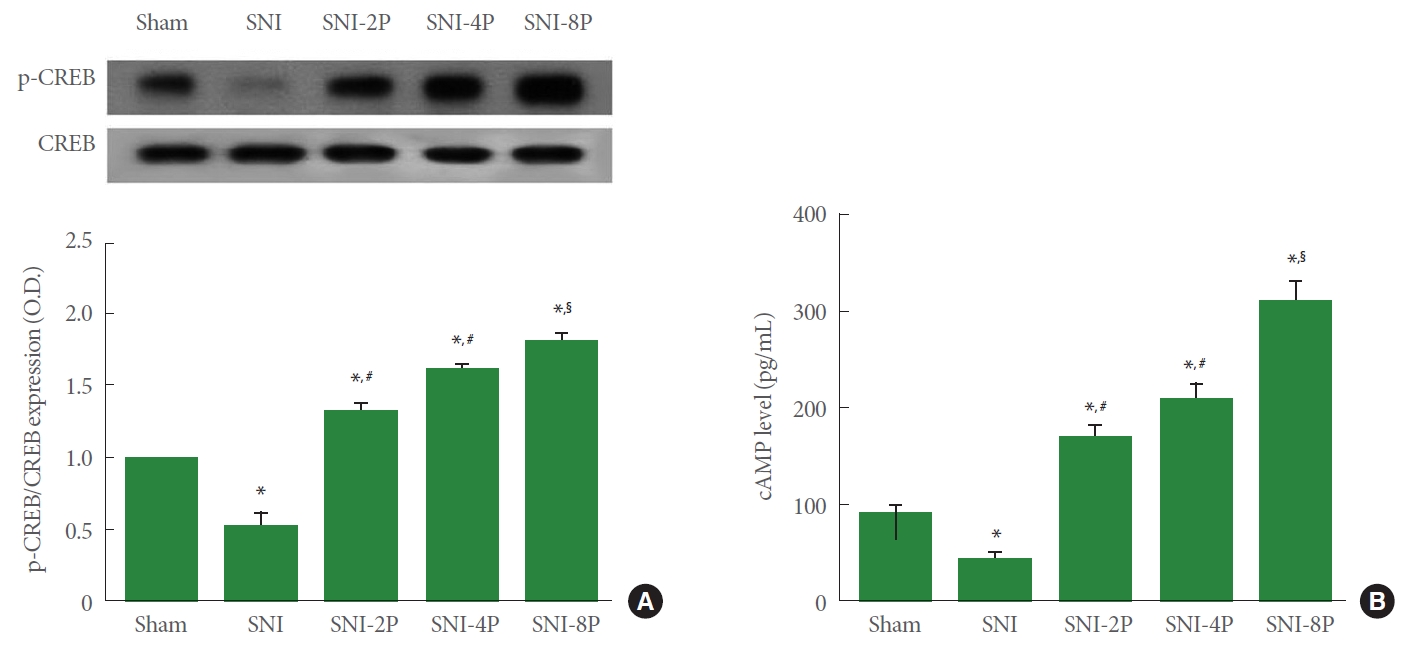
Effect of PDRN treatment on the ratio of p-CREP to CREP and cAMP level in sciatic nerve tissues. (A) CREP expression. Upper panel: representative expression of CREP. Lower panel: ratio of p-CREP to CREP. (B) cAMP expression. Sham, Sham operating group; SNI, SNI inducing group; SNI-2P, SNI inducing with 2-mg/kg PDRN treating group; SNI-4P, SNI inducing with 4-mg/kg PDRN treating group; SNI-8P, SNI inducing with 8-mg/kg PDRN treating group. PDRN, polydeoxyribonucleotide; CREP, cAMP response element binding protein; p, phosphorylated; cAMP, cyclic adenosine-3´,5´-monophosphate; SNI, sciatic nerve crush injury. *P<0.05 compared to the sham operating group. #P<0.05 compared to the SNI inducing group. §P<0.05 compared to the 2 mg/kg PDRN treating group.
In ELISA analysis, cAMP expression was inhibited in SNI-induced rats (P<0.05), however, treating with PDRN increased expression of cAMP in sciatic nerve tissues. In particular, 8-mg/kg PDRN treatment more strongly increased cAMP level (P<0.05).
VEGF Expression
Fig. 5. shows VEGF expression calculated by western blot, Induction of SNI resulted in a decrease in VEGF expression in sciatic nerve tissues (P<0.05). However, treating with PDRN overexpressed VEGF. In particular, 8-mg/kg PDRN treatment more strongly increased VEGF expression (P<0.05).
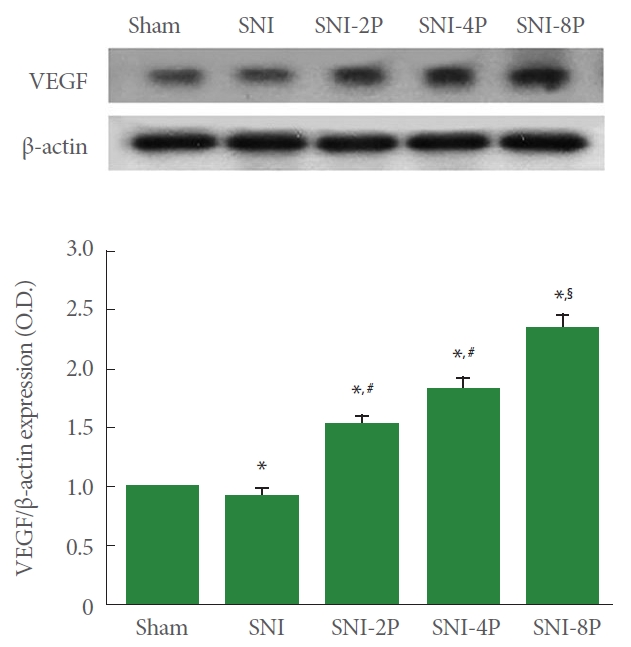
Effect of PDRN treatment on VEGF expression in sciatic nerve tissues. (A) VEGF expression. Upper panel: Representative expression of VEGF. Lower panel: Relative expression of VEGF. Sham, Sham operating group; SNI, SNI inducing group; SNI-2P, SNI inducing with 2-mg/kg PDRN treating group; SNI-4P, SNI inducing with 4-mg/kg PDRN treating group; SNI-8P, SNI inducing with 8-mg/kg PDRN treating group. PDRN, polydeoxyribonucleotide; VEGF, vascular endothelial growth factor; SNI, sciatic nerve crush injury. *P<0.05 compared to the sham operating group. #P<0.05 compared to the SNI inducing group. §P<0.05 compared to the 2 mg/kg PDRN treating group.
Neurofilament Expression
Neurofilament expression detected by immunofluorescence is shown in Fig. 6. Induction of SNI decreased neurofilament expression in sciatic nerve tissues. In contrast, PDRN treatment enhanced neurofilament expression.
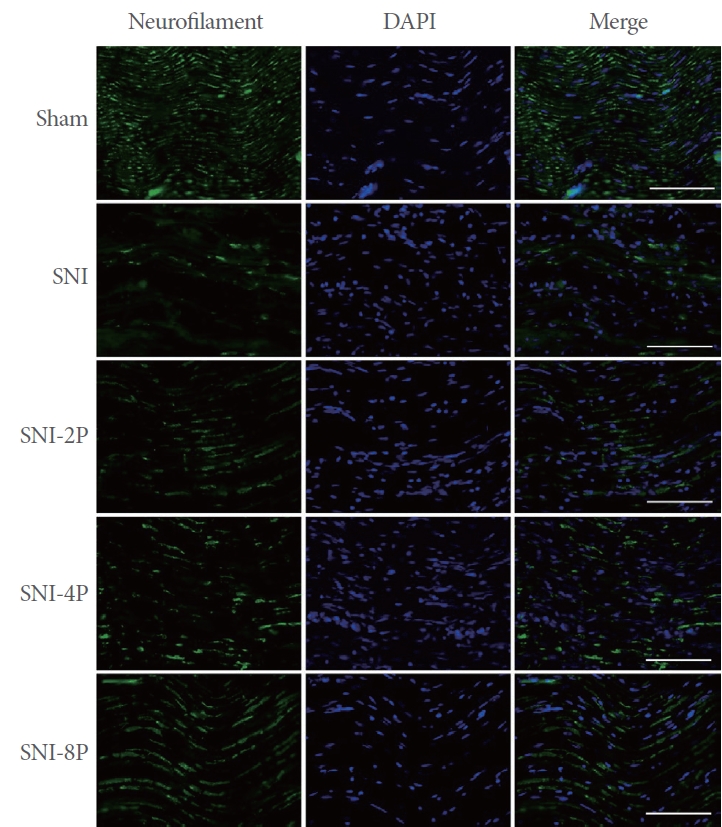
Effect of PDRN treatment on neurofilament expression. The expression of neurofilament-immunoreactive fibers in the sciatic nerve tissues. The scale bars represent 200 μm. Sham, Sham operating group; SNI, SNI inducing group; SNI-2P, SNI inducing with 2-mg/kg PDRN treating group; SNI-4P, SNI inducing with 4-mg/kg PDRN treating group; SNI-8P, SNI inducing with 8-mg/kg PDRN treating group. PDRN, polydeoxyribonucleotide; DAPI, 4´,6-diamidino-2-phenylindol; SNI, sciatic nerve crush injury.
DISCUSSION
The widely used index SFI is for the determination of the walking function after peripheral nerve lesions [20]. An increase in SFI indicates an improvement in locomotor function, and a decrease in SFI indicates a worsening of locomotor function. The current results showed that rats that received SNI demonstrated a typical pattern of footprint indicative of decreased SFI value. Rats in the PDRN treatment groups showed increased SFI value. Treatment with 8-mg/kg PDRN showed the greatest increment in SFI value. The results of this experiment show that PDRN treatment improved motor function after SNI.
Peripheral nerve damage induces neuropathic pain which characteristic symptoms of allodynia and hyperalgesia. It is known that cold allodynia and heat hyperalgesia were induced in rats receiving sciatic nerve injury [21]. In this study, pain measurement using the plantar test showed that SNI significantly increased thermal sensitivity. SNI also enhanced the expression of TNF-α and IL-1β, factors involved in inflammatory processes. Many studies have reported that the inflammatory response during nerve damage is responsible for the basic mechanism of neuropathic pain development [22-24]. Neuropathic pain was caused by intraneural insertion of TNF-α and IL-1β [25]. The production of TNF-α and IL-1β is essential for the induction of neuropathic pain, and therefore anti-TNF-α or anti-IL-1β treatment is very effective in suppressing the occurrence of neuropathic pain [22].
Activation of the adenosine A2A receptor, expressed on most cells implicated in healing of wound, increases intracellular cAMP levels and inhibits inflammatory neutrophil function. Adenosine A2A receptor activation promotes CREP phosphorylation process through the cAMP-protein kinase A pathway, and this phosphorylation of CREP regulates nuclear factor-κB, thereby inhibits proinflammatory cytokines’ secretion [26]. The results of this experiment demonstrated that PDRN treatment caused cAMP overexpression in SNI-induced rats, and this increased cAMP expression suppressed proinflammatory cytokines’ production in sciatic nerve tissues. PDRN has proven efficacy as a therapeutic modality for inflammatory diseases [27].
Another important feature of peripheral nerve injury is changes in neurofilament and VEGF expression. Neurofilaments are abundant in axonal processes and involved in axonal transport and cell shape maintenance [28]. However, neurofilament activation is reduced due to various injuries to the peripheral nervous system. Angiogenesis acts a critical role in wound healing, and VEGF is known to strongly stimulate angiogenesis. [17]. During the peripheral nerve healing process, microvessels and capillary networks within the tissue are formed under the influence of VEGF [29]. Expression of VEGF, which is involved in nociceptive signaling and regeneration, is induced by stimulation of adenosine A2A receptors [17]. At the skin incision site, PDRN stimulates adenosine A2A receptors to promote VEGF production and fibroblast maturation, thereby speeding up the healing process [30]. In this study, VEGF and neurofilament expression were decreased by SNI. In contrast, PDRN treatment overexpressed VEGF and enhanced neurofilament expression, suggesting that PDRN promotes regeneration of sciatic nerve injury.
In the current study, treatment with PDRN was shown to improv motor function by suppressing the inflammatory response and pain generation induced by SNI. Overexpression of VEGF through the CREP-protein kinase A pathway may be proposed as the underlying mechanism of PDRN during nerve healing. Therefore, it is believed that PDRN can be used as an effective new agent for neuropathic pain induced by SNI associated with the lithotomy position after urologic surgery.
Notes
Grant/Fund Support
This study was supported by a grant from the National Research Foundation of Korea (NRF-2021R1F1A1057202).
Research Ethics
This experimental procedure was conducted after approval from the Kyung Hee University’s ethics committee and the approval number was KHUASP(SE)-21-348.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTION STATEMENT
· Conceptualization: JHH, JYC, SOK
· Data curation: JHH, YCJ, JYC
· Formal analysis: JHH, YCJ, SOK
· Funding acquisition: JHH
· Methodology: YCJ, JYC, SOK
· Project administration: JHH
· Visualization: JHH, YCJ, SOK
· Writing – original draft: YCJ
· Writing – review & editing: JHH