The Risk of Stress Urinary Incontinence After Hysterectomy for Uterine Fibroids
Article information
Abstract
Purpose
We evaluated the relationship between previous hysterectomy for uterine fibroids and subsequent stress urinary incontinence (SUI).
Methods
This study analyzed national health insurance data. The hysterectomy group (aged 40 to 59) comprised patients who underwent hysterectomy for uterine fibroids between January 1, 2011 and December 31, 2014, and the control group (aged 40 to 59) comprised patients who visited a medical facility for a checkup during the same time span. One-to-one propensity score matching was performed to balance confounders. SUI was defined as the need for SUI surgery accompanied by a diagnosis code for SUI.
Results
After matching, 81,373 cases (hysterectomy group) and 81,373 controls (nonhysterectomy group) were enrolled. The mean follow-up period was 7.9 years for the cases and 7.8 years for the controls. The incidence of anti-incontinence surgery was slightly but significantly higher in the cases than in the controls (2.0% vs. 1.7%, P<0.001). Compared to the control group, abdominal hysterectomy significantly increased the likelihood of anti-incontinence surgery both before (hazard ratio [HR], 1.235; 95% confidence interval [CI], 1.116–1.365) and after adjusting for confounders (HR, 1.215; 95% CI, 1.097–1.347). In contrast, laparoscopic hysterectomy, laparoscopic hysterectomy with adnexal surgery, and abdominal hysterectomy with adnexal surgery were not associated with an increased rate of anti-incontinence surgery. The significant association between abdominal hysterectomy and an elevated rate of anti-incontinence surgery persisted even after stratifying patients by age group.
Conclusions
Prior abdominal hysterectomy without adnexal surgery was associated with an increased incidence of subsequent anti-urinary incontinence surgery.
INTRODUCTION
Uterine fibroids (myomas or leiomyomas) are the most prevalent benign tumors of the uterus. In South Korea, the incidence of uterine fibroids has surged, with their prevalence in 2022 being 2.5 times greater than it was in 2013 [1].
Uterine fibroids can cause various symptoms, including pelvic pain, abnormal uterine bleeding, and dysmenorrhea, all of which necessitate treatment [1]. Hysterectomy is the most effective treatment for symptomatic uterine fibroids, and uterine fibroids are currently the most common indication for hysterectomy worldwide [2,3].
Previous studies have investigated the relationship between previous hysterectomy for benign conditions and subsequent stress urinary incontinence (SUI) [4-8]. However, findings have been inconsistent across these studies. There is also a notable lack of long-term follow-up data from large-scale studies [3]. Furthermore, the impact of prior hysterectomy for benign reasons on the need for subsequent SUI surgery has seldom been examined [8], and to our knowledge, there is only one dataset available on this topic. This issue is particularly significant given the high prevalence of SUI, its association with poor hygiene and diminished quality of life [1,9], and the fact that the most common surgical treatment for SUI, the midurethral sling using mesh, may lead to complications such as pain, erosion, and infection [10].
Therefore, we conducted this study to investigate the relationship between prior hysterectomy for uterine fibroids and the subsequent risk of surgery for SUI, utilizing nationally representative data with long-term follow-up.
MATERIALS AND METHODS
Database
South Korea provides public health insurance to all its citizens [11]. As a result, the National Health Insurance Service (NHIS) of Korea has the capability to access the medical records of the majority of the population, which is approximately 51 million people. These records include details such as sex, age, name of surgery, prescription drug names, diagnosis names, type of medical insurance, and records of hospitalization and outpatient treatment. The Health Insurance Review and Assessment Service (HIRA) is a national entity responsible for arbitrating health insurance payments between the NHIS and medical institutions. Consequently, HIRA has access to a vast amount of medical record information from the National Health Insurance Corporation pertaining to Koreans. The present population-based retrospective cohort study analyzed health insurance data from HIRA, spanning from January 1, 2007 to December 31, 2020.
Selection of Participants
The International Classification of Diseases, 10th Revision and the Korea Health Insurance Medical Care Expenses (2016, 2019 edition) were used for the analyses in this study. The hysterectomy group included women aged 40 to 59 who underwent a hysterectomy for uterine leiomyoma or adenomyosis from January 1, 2011 to December 31, 2014. The date of the hysterectomy was set as the inclusion date. Hysterectomy and adnexal surgery were considered to be performed concurrently if adnexal procedures (such as oophorectomy, salpingo-oophorectomy, salpingectomy, ovarian cystectomy, adnexectomy, incision and drainage of ovarian cysts, or ovarian wedge resection) occurred on the same day as the hysterectomy. The selection of the hysterectomy approach for uterine fibroids typically depends on their location, size, and number. The control group comprised women aged 40 to 59 who visited a medical facility for a checkup during the same period. Those who had undergone a hysterectomy were excluded from the control group. The inclusion date for the control group was the date of the first health examination visit. In both groups, patients with any form of cancer (any Cxx code) or urinary incontinence (codes N39.3 for stress incontinence, N39.40 for urge incontinence, N39.41 for mixed incontinence, and N39.48 for other specified urinary incontinence) were excluded if these conditions were diagnosed before the 180th day after inclusion. For the selected hysterectomy and control groups, 1:1 propensity score matching was conducted. This matching accounted for age in 5-year intervals, year of inclusion, socioeconomic status (SES), parity, region, Charlson comorbidity index (CCI), any adnexal surgery prior to inclusion, menopause status before inclusion, use of menopausal hormone therapy, and any history of pelvic organ prolapse before inclusion.
Outcome
SUI was defined as the occurrence of SUI surgery—specifically, procedures via a transvaginal approach (R3564, R3565), an abdominal approach (R3562), or injections of foreign material or autologous fat (R3563)—accompanied by a urinary incontinence diagnosis code (N39.3, N39.4).
Variables
Areas outside a metropolitan region were classified as rural. SES was considered low when the individual’s health insurance was categorized as Medical Aid. The CCI was calculated using diagnostic codes from the year preceding the inclusion date up to the date of inclusion. Parity was assessed by identifying the number of deliveries that occurred within the study period. Adnexal surgery was characterized as having undergone at least one adnexal procedure prior to hysterectomy. Menopause was determined by at least 2 recorded visits for menopausal conditions (N95.x, menopausal and other perimenopausal disorders; M80.0, postmenopausal osteoporosis with pathological fracture; M81.0, postmenopausal osteoporosis; E28.3, premature menopause) before undergoing hysterectomy. Patients were considered to have been on menopausal hormone therapy prior to inclusion if they were prescribed any of the following medications at least 180 days before the inclusion date: tibolone, estradiol hemihydrate, estradiol valerate, dydrogesterone, norethisterone acetate, drospirenone, medroxyprogesterone acetate, or cyproterone.
Statistics
In this study, SAS Enterprise Guide 7.15 (SAS Institute Inc., Cary, NC, USA) and R 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria) were employed for statistical analysis. A P-value of 0.05 or lower was deemed statistically significant for all analyses, with a 2-sided test being conducted. The Cochran-Mantel-Haenszel test was used for the analysis of categorical variables, while the Wilcoxon signed-rank test was applied to continuous variables. Standardized differences were used to evaluate matched variables.
After adjusting for confounding variables, we employed a stratified Cox regression model to assess the risk of SUI associated with hysterectomy. The start date for the Cox analysis was the inclusion date for each group, while the end date was either the date of death or December 31, 2020. For handling missing data, we applied the pairwise deletion method when the percentage of missing values was below 10%. Conversely, when the percentage exceeded 10%, we used the regression imputation method. To validate the findings of our study, we conducted a stratified Cox regression analysis on the risk of SUI following laparoscopic hysterectomy.
Ethics
The Institutional Review Board (IRB) of Sanggye Paik Hospital approved this research (IRB No. SGPAIK 2021-12-005). In this study, any identifiers that could be used to recognize individuals were removed in accordance with the HIRA’s personal protection policy. The analysis was performed exclusively on HIRA’s secure server. Only the final data values were extracted from the server. Due to these precautions, there was no risk of participant identification, and as such, informed consent was deemed unnecessary under the Bioethics and Safety Act of South Korea. According to HIRA’s privacy policy, only the research findings can be exported from the server, which precludes the sharing of raw data. While this study made use of HIRA data, HIRA has no vested interest in the research.
RESULTS
After excluding participants who had undergone surgery for SUI either before 180 days following their initial medical examination or subsequent to a hysterectomy, the study ultimately included a total of 86,722 patients who had undergone hysterectomy for uterine fibroids and 135,680 women who had not (Fig. 1). Following 1:1 propensity score matching, the final cohort consisted of 81,373 cases in the hysterectomy group and an equal number of controls in the nonhysterectomy group. The average follow-up period was 7.8 years for the nonhysterectomy group and 7.9 years for the hysterectomy group. Post-matching, all variables exhibited a well-balanced distribution, with a standardized difference below 0.1 in the propensity-weighted cohort. The characteristics of the matched participants are detailed in Table 1.

Flowchart of the study selection process for women according to hysterectomy status from the National Health Insurance Database, 2007–2020.

The characteristics of women with/without hysterectomy obtained from the National Health Insurance Database, 2007–2020
Anti-incontinence surgery was modestly yet significantly more common among the cases (n=1,612) compared to the controls (n=1,401), corresponding to rates of 1.7% versus 2.0%, respectively (P<0.001).
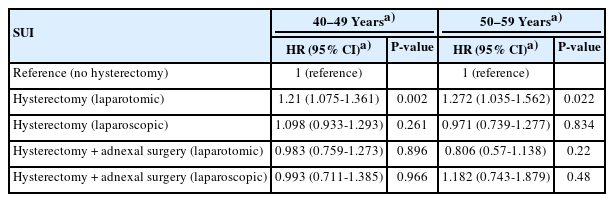
Table 2 presents the risk of requiring anti-incontinence surgery based on the type of hysterectomy performed. When compared with the nonhysterectomy group, the rate of anti-incontinence surgery was significantly higher in patients who underwent abdominal hysterectomy, both before and after adjusting for confounding factors. In contrast, laparoscopic hysterectomy, laparoscopic hysterectomy with adnexal surgery, and abdominal hysterectomy with adnexal surgery did not show an increased rate of anti-incontinence surgery relative to the nonhysterectomy group, with adjustments for confounders taken into account. The notable association between abdominal hysterectomy and an elevated rate of anti-incontinence surgery persisted even after stratifying the patients by age group, as indicated in Table 3.
DISCUSSION
In this study, undergoing a hysterectomy for the treatment of uterine fibroids was associated with a modest yet significant increase in the likelihood of requiring subsequent anti-incontinence surgery. Of all the surgical types considered, only abdominal hysterectomy without concurrent adnexal surgery showed an association with the need for anti-incontinence procedures.
SUI is the most common type of urinary incontinence [12]. It is characterized by involuntary urine leakage prompted by actions such as coughing, sneezing, or physical exertion that increase abdominal pressure. Without treatment, SUI can disrupt daily life and diminish quality of life. Behavioral strategies and conservative management, which include weight reduction and pelvic floor muscle exercises, are recommended as initial treatments. For women with SUI who do not respond to conservative measures, surgical intervention, such as the placement of midurethral slings, may be considered [13]. The most common surgical procedure for SUI involves the insertion of a midurethral sling via a transobturator or retropubic approach. However, this surgery carries risks of complications, including pelvic hematoma, bladder perforation, urinary retention, pain, erosion, and infection [10].
Previous studies have explored the link between hysterectomy and overall urinary incontinence [4-8]. However, the findings have been inconsistent, and the validity of these studies is limited by factors such as the small sample sizes [5], the relatively short follow-up period of 1 year [6], and potential recall bias [4]. Furthermore, there is a paucity of data on the relationship between SUI and hysterectomy from long-term cohort studies or randomized controlled trials, with results that also vary between studies.
A French cohort study, with a mean follow-up of 4.6 years, found no increased risk of SUI after vaginal hysterectomy for benign conditions, including 1,171 cases of hysterectomy for menorrhagia, when compared to a control group [4]. However, the study’s reliance on a mail survey to collect data could lead to recall bias, and it is worth noting that the response rate was only about 50%. In another cohort study that followed 371 cases of hysterectomy over 10 years, it was shown that hysterectomies performed for menorrhagia or dyspareunia did not significantly raise the incidence of SUI when compared to laparoscopic cholecystectomy or transcervical endometrial resection [5]. The validity of these findings, however, is limited by the relatively small sample size.
A randomized controlled study from the United Kingdom demonstrated a decrease in SUI as observed in a urodynamic study following total abdominal hysterectomy for benign conditions. Nevertheless, these results are limited by the relatively short follow-up period of 1 year [6].
Contrary to the findings of other studies, a large observational study from the US reported a significantly higher incidence of SUI 3 years post-hysterectomy compared to controls (odds ratio, 1.23; 95% confidence interval [CI], 1.11–1.36), with 53,569 individuals in the control group and 38,524 in the hysterectomy group [7]. Furthermore, a nationwide, population-based cohort study from Sweden found that hysterectomy for benign conditions was associated with an increased risk of subsequent SUI surgery relative to controls (hazard ratio, 2.4; 95% CI, 2.3–2.5) [8]. These findings are consistent with our results. Our study also corroborates the Swedish cohort study’s findings [8] using an alternative dataset. Given that anti-incontinence surgery is typically reserved for women with more severe SUI, carries a range of complications, and incurs higher costs than other SUI treatments, we believe our data, alongside the Swedish findings, hold significant clinical relevance among the existing literature examining the link between SUI and hysterectomy for benign reasons. The findings of this study indicate that patients considering transabdominal hysterectomy for the treatment of uterine fibroids should be counseled about the potential increased risk of SUI, particularly the heightened risk of requiring anti-incontinence surgery.
The reason for the increased incidence of anti-incontinence surgery following hysterectomy remains unclear, and investigating the underlying mechanism within a study setting presents challenges. Hysterectomy may cause damage to the distal branches of the inferior hypogastric and pudendal nerves, potentially disrupting the function of the urethral sphincter [14]. This could account for the findings observed in our results.
In a subanalysis, only transabdominal hysterectomy without concurrent adnexal surgery was associated with subsequent anti-incontinence surgery. Conversely, laparoscopic hysterectomy showed no such association, regardless of whether adnexal surgery was performed. The enhanced visualization provided by laparoscopy may facilitate precise dissection that spares surrounding tissues, including the pelvic autonomic fibers. Furthermore, transabdominal hysterectomy is typically the chosen approach for larger uterine fibroids [15]. It can be deduced that the excision of large uterine fibroids might result in more significant nerve and tissue damage compared to the removal of smaller fibroids. Consequently, the incidence of anti-incontinence surgery is likely to be higher in patients undergoing transabdominal hysterectomy than in those undergoing laparoscopic hysterectomy.
In terms of adnexal surgery, preserving the adnexa in patients undergoing abdominal hysterectomy may be associated with higher serum estrogen levels compared to those who have both an abdominal hysterectomy and adnexal surgery. This is because retaining the adnexa during a hysterectomy could potentially delay the onset of menopause, as indicated by sustained serum estrogen levels. A previous, well-designed clinical trial found that estrogen replacement therapy increased the incidence of SUI in women who had a hysterectomy [16]. This finding implies that elevated serum estrogen levels might heighten the risk of SUI in women post-hysterectomy. In this study, the impact of transabdominal hysterectomy, which can damage nerves and surrounding tissues, on SUI, combined with the influence of adnexal preservation (and consequently higher estrogen levels), appears to contribute to an increased incidence of SUI in patients who underwent abdominal hysterectomy without adnexal surgery.
In this study, undergoing a laparoscopic hysterectomy without concurrent adnexal surgery was not associated with antiincontinence procedures. It is possible that laparoscopic hysterectomy could reduce the risk of SUI due to the preservation of pelvic autonomic fibers, while the preservation of the adnexa might increase the risk of SUI due to elevated estrogen levels, as previously reported. Consequently, the potential positive and negative effects of laparoscopic hysterectomy without adnexal surgery on SUI may offset each other. Additional follow-up data are required to explore the relationship between adnexal surgery and SUI.
It is necessary to acknowledge the limitations of this study. First, we were unable to determine whether patients underwent a bilateral or unilateral salpingo-oophorectomy, as these specific procedures are not itemized in the HIRA dataset with distinct operative codes. Second, the HIRA database does not provide detailed information on the disease, such as the symptoms or severity of uterine fibroids, nor does it include data on obesity, a known risk factor for SUI. Despite these limitations, our study is based on a large, population-based cohort with an extensive follow-up period. Consequently, we believe that our findings are still of significant value.
In conclusion, prior abdominal hysterectomy without concurrent adnexal surgery was associated with a higher incidence of subsequent antiurinary incontinence surgery.
Notes
Research Ethics
The Institutional Review Board (IRB) of Sanggye Paik Hospital approved this research (IRB No. SGPAIK 2021-12-005).
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTION STATEMENT
· Conceptualization: JSY, JHL
· Data curation; JSY, ICC, JHL
· Formal analysis: ICC, JHL
· Funding acquisition: JSY, JHL
· Methodology: ICC, JHL
· Project administration: JSY
· Visualization: ICC
· Writing - original draft: JSY, JHL
· Writing - review & editing: JSY, ICC, JHL