Detrusor Overactivity After Partial Bladder Outlet Obstruction Is Associated With High Urinary Adenosine Triphosphate Levels in Female Wistar Rats
Article information
Abstract
Purpose
Bladder outlet obstruction (BOO) commonly causes detrusor overactivity (DO). In this study, a post hoc analysis of previous obtained data, we investigate if DO occurring in initial phases of BOO is associated with changes in urinary adenosine triphosphate (ATP) levels.
Methods
Adult female Wistar rats were submitted to partial BOO (pBOO) or to sham obstruction. Cystometry was performed at 3 or 15 days after pBOO and saline voided was collected for ATP determination. Normality was tested using Shapiro-Wilk test. The mean frequency of voiding contractions (VCs) of the sham-operated animals at 15 days after surgery, plus or minus 3 standard deviations, was used to represent the normal range. Statistical analyses were performed using the chi-square and Mann-Whitney tests.
Results
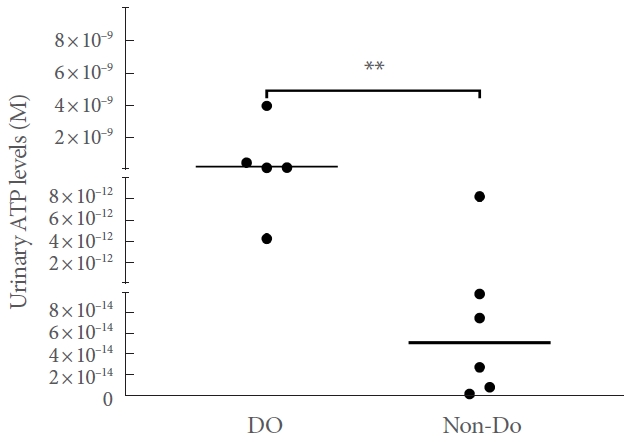
DO was indicated by a VC frequency greater than or equal to 0.9 VCs/min. DO was observed in 63% of animals at 3 days and in 33% at 15 days following pBOO. ATP levels were significantly higher in rats with DO compared to those without DO.
Conclusions
The DO phenotype, occurring in the initial phases of BOO, is associated with comparatively high urinary ATP levels.
• HIGHLIGHTS
- Detrusor overactivity, which prevails in early stages of partial bladder outlet obstruction concurs with high urinary adenosine triphosphate levels that may reflect urothelial compensatory mechanisms.
INTRODUCTION
Detrusor overactivity (DO) associated with partial bladder outlet obstruction (pBOO) is a common event among men with enlarged prostate glands [1] that usually precedes detrusor underactivity (DU), a feared terminal event that affects a smaller proportion of men with chronic bladder outlet obstruction (BOO) [2].
The EpiLUTS (Epidemiology of Lower Urinary Tract Symptoms) survey has revealed that among males above the age of 40 years, storage symptoms are more prevalent than voiding symptoms [3]. Among 1,418 men with a median age of 63 years investigated urodinamically due to benign prostatic obstruction (BPO)-lower urinary tract symptoms, 60.9% had DO [1]. This suggests that bladder storage symptoms are a significant problem to deal with in men with BPO and pBOO.
In response to obstruction, the bladder undergoes compensatory mechanisms to strengthen detrusor contraction, with detrusor hypertrophy being the most prominent adaptation [4, 5]. Additionally, changes in cell-to-cell connectivity and neurotransmitter leakage from impaired parasympathetic nerve terminals can lead to detrusor micromotions [6]. An excess of afferent activity may occur due to sprouting of sensory fibers in the sacral spinal cord [7]. The urothelium, in response to increased intravesical pressure expected at least during the initial phases of pBOO increase the release of urothelial adenosine triphosphate (ATP) [8, 9]. An interesting aspect of the potential role of ATP, is the stimulation of voiding reflex after binding to P2X3 purinergic receptors expressed in the rich sub-urothelial sensory fiber network, which could play a key role in the development of DO following pBOO [9-11].
This study aims to explore the hypothesis that DO observed in the initial phases of pBOO is associated with high urinary levels of ATP. This aspect became relevant following the development of P2X3 specific antagonists that could be useful to treat ATP mediated DO [12].
MATERIALS AND METHODS
Animals
This study is a subanalysis of a previous study in which BOO was induced in female rats to investigate the levels of urinary ATP and the incidence of DU in the initial stage of BOO and if deobstruction would revert such phenotype [13]. Here only animals which had DO at the time points established for analysis, 3 and 15 days after the induction of pBOO, are considered. We have used female, instead of male, due to methodological limitation.
Arrive guidelines were followed. Female Wistar rats aged between 3 to 4 months were utilized in this experiment. The rats were housed in type IV cages with access to tap water ad libitum. The housing conditions included an inverted light cycle, a controlled temperature of 22°C–24°C and humidity maintained at 45%–65%.
Partial Bladder Outlet Obstruction
pBOO was performed following a previously established protocol [14], with animals under isoflurane anesthesia (4% for induction and 1.5% for maintenance). Briefly, a midline suprapubic incision was made to expose the bladder neck and urethra. A catheter was inserted, and the urethra was dissected to create a dorsal passage. A 2-0 silk suture was passed dorsally and tied around the entire urethra and a metal rod with an outside diameter of 1.0 mm. Subsequently, the catheter and metal rod were removed. For the control group, the urethra was exposed and dissected in a similar manner, but no silk suture was left in place.
Following the surgical procedure, animals received 20 mg/kg of tramadol hydrochloride orally for 2 consecutive days. Postsurgery, animals were monitored daily. If blood was observed in the urine and animals presented 3 or more humane endpoint signs, they would be immediately euthanized with an intraperitoneal injection of 150 mg/kg of sodium pentobarbital. Two animals died before meeting these endpoints and were not included in the experimental groups. The first animal died during the anesthetic induction, and the second one died 7 days after surgery due to an unknown cause.
Cystometries and DO Definition
Cystometries were performed to assess the frequency of voiding contractions (fVC), measured as bladder contractions per minute, which, in the absence of validated pressure-flow studies, has been used as a surrogate marker of DO in rats [11, 15]. From a statistical point of view, it is expected that the majority of sham population have mean fVC±3 standard deviation (SD). In this experiment, we defined BOO animals as having DO phenotype if their fVC was plus 3 SDs of the mean of fVC observed in sham-operated animals at 15 days after the sham surgery. All other animals were considered as having non-DO phenotype.
Cystometries were performed under urethane anesthesia (1.2-g/kg body weight), and the rats laid on a heating pad with a rectal probe to maintain body temperature constant at 37°C (WPI ATC 1000 DC, World Precision Instruments Inc., FL, USA). The bladder was exposed through a low abdominal incision, and a 20-gauge needle was inserted into the bladder dome. Saline was infused at a rate of 6mL/hr using a WPI SP100IZ syringe pump (World Precision Instruments Inc.). After a stabilization period of 30 minutes, the fVC was recorded for 10 minutes. Fluid collected from the urethra during cystometries, resulting from spontaneous voiding or overflow, was stored at -80°C for later ATP determination.
ATP Measurement
Urinary ATP levels (molar, M) were determined by luminometry, using the ATP Bioluminescence Assay Kit HSII (Sigma-Aldrich, Lisbon, Portugal). The assay involved adding luciferase to the well of a 96-well white plate, followed by an equal volume of the urine sample. The highest luminescence value obtained from each sample was recorded. To ensure accuracy and minimize potential interference from urinary compounds, the lowest luminescence reading was stabilized before conducting a standard addition. This step helped to accurately quantify the ATP levels in the urine samples.
Statistical Approach and Sample Size
The statistical analysis in this study involved a post hoc analysis of data obtained from previous experiments [13]. Sample size was determined based on the previous study [13]. To ensure adequate statistical power and to detect meaningful differences between groups, we considered an effect size=1.2, α=0.05, power=0.95 for 4 groups and determined 5 animals/group. However, as pBOO did not induce consistently a DU phenotype at different time points, we used 10 animals at day 3 and day 15. As 2 animals for analysis at 3 days after pBOO and 1 animal at 15 days after pBOO died, these groups had 8 and 9 animals, respectively.
Normal distribution was assessed using Shapiro-Wilk test. To analyze the change in the proportion of animals with DO and non-DO at studied experimental timepoints, a chi-square test was used. The urinary levels of ATP of animals with DO and non-DO phenotype were compared using Mann-Whitney test (GraphPad Software Inc., La Jolla, CA, USA). The significance level was set at P<0.05.
RESULTS
Urodynamic Phenotype After pBOO
The fVC of sham-operated animals (n=5) follow a normal distribution (Shapiro-Wilk test; P=0.3140). In sham-operated animals (n=5), the mean fVC was 0.6±0.1 contractions/minute. From a statistical point of view, it is expected that the majority of normoactive population have fVC 0.6±3 SD. In other words, it is expected that their fVC fall in the interval between 0.3 and 0.9 contractions per minute. Based on this pre-established definition, the DO phenotype was set at fVC≥0.9 VC/min, and non-DO at fVC<0.9 VC/min (being 0.3<fVCnormoactive<0.9 and fVCDU ≤0.3). The proportion of animals with DO phenotype 3 days after pBOO (5 out of 8 animals; 63%) was higher than 15 days after pBOO was (3 out of 9 animals; 33%) (chi-square analysis, P<0.0001) (Fig. 1). Therefore, the proportion of animals with non-DO phenotype 3 days after pBOO (1 out of 8 animals with DU and 2 out of 8 animals normoactive; 37%) was lower than 15 days after pBOO was (6 out of 9 animals with DU; 67%) (chi-square analysis, P<0.0001) (Fig. 1).
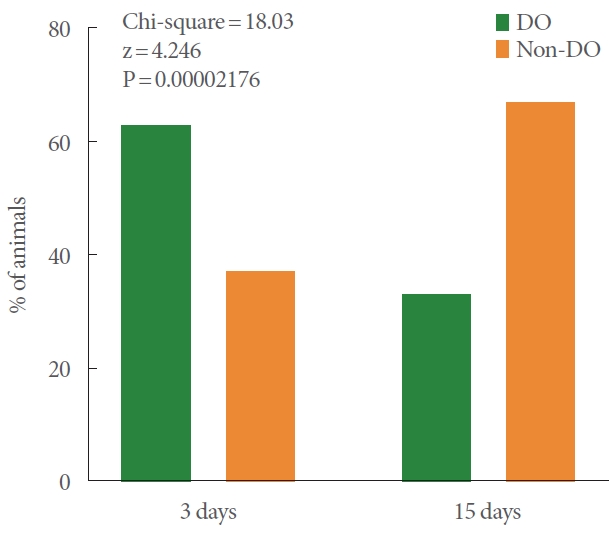
Interleaved bar graph displaying the percentages (%) of animals exhibiting DO and non-DO phenotypes at 3 and 15 days after pBOO. DO, detrusor overactivity; pBOO, partial bladderoutlet obstruction.
The fVC of animals with DO phenotype after pBOO (n=8) follow a normal distribution (Shapiro-Wilk test; P =0.6442). These animals had a mean fVC=1.0±0.3 contraction/min.
Urinary ATP Levels
Due to technical problems, it was not possible to assess the levels of ATP in 3 samples from animals with DO and 3 samples from animals with non-DO phenotype after pBOO (ee raw data in the Supplementary Material). The urinary levels of ATP in DO and non-DO animals did not follow a normal distribution (Shapiro-Wilk test; P=0.0009 and P<0.0001, respectively). Animals with DO phenotype after pBOO (n=5) had more urinary ATP than animals with non-DO phenotype after pBOO (n=6) (Mann-Whitney test; P=0.0087) (Fig. 2).
DISCUSSION
The most important finding of this post hoc analysis is the association between DO phenotype induced by pBOO and high urinary ATP levels. This suggests that the increased urinary ATP levels may trigger bladder voiding contractions, possibly as a urothelium/suburothelium crosstalk as a response to the obstruction. ATP released by urothelial cells act on urothelial P2X3 receptor that works as a paracrine amplifier of ATP effects, promoting the firing of suburothelial afferents that lead to bladder contractions [16]. ATP can also trigger P2X3 receptors expressed in suburothelial nerve fibers [9], favoring bladder contractions. ATP released by urothelial cells may also reach the urine through a pannexin 1 channels-mediated mechanism, where it seems to play a role in the control of bladder activity [17]. Hence, in the present work, the observed increase in urinary ATP reflects the increase in its release by urothelial cells and, consequently, the possible activation of downstream events including the P2X3 receptor-mediate bladder contractions. In fact, previous studies showed that men with BOO, in which DO was not systematically investigated, there was a positive correlation between urinary ATP levels and BOO index [18, 19]. Nevertheless, the present study should be replicated in man with DO following BOO to understand if these patients also present changes in their urinary ATP levels compared to healthy subjects.
This study also showed the importance of duration of pBOO and the presence of DO phenotype. While in rats, 3 days of pBOO led to DO in about 2/3 of the animals, at 15 days DO was present in 1/3 of the animals. The remaining, at 15 days had a DU phenotype indicating a severe deterioration of the bladder function, as previously reported [13]. The early predominance of DO phenotype is in accordance with the concept that, to overcome obstruction, bladders develop swiftly compensatory mechanisms. In addition to the increase of ATP released from the bladder urothelium, one cannot ignore that other mechanisms may play important roles. Examples include the increase of nerve growth factor levels in the bladder wall, enhancing the excitability of bladder sensory C-fibers, sprouting of bladder sensory fibers in the sacral spinal cord, and development of abnormal contact between detrusor smooth muscle cells [20, 21]. Additionally, it was hypothesized that neurotransmitter leakage from impaired parasympathetic nerve terminals can lead to detrusor micromotions [6].
This study may have relevant clinical implications, now that P2X3 antagonists are available [12]. Gefapixant, a P2X3 antagonist, was approved for treatment of refractory chronic cough or unexplained chronic cough [22]. Gefapixant was tested to treat woman with moderate to severe pain associated with Interstitial Cystitis/Bladder Pain Syndrome (IC/BPS) (ClinicalTrials.gov ID NCT01569438). This drug proved to decrease pain and urinary urgency on those patients compared to placebo. Although it is not known whether this work will improve lower urinary tract symptoms in patient with storage symptoms and BOO, there is perspective that this may occur as both BOO and IC/BPS seem to rely on the activation of P2X3 receptors to drive bladder changes observed on those patients [23, 24]. In fact, in animal models of cyclophosphamide (CYP)-induced cystitis, P2X3 antagonist A-317491 improved the bladder overactivity induced by CYP [25]. Also, the endogenous Pirt protein regulates P2X3 activity, reducing bladder overactivity [26]. Another P2X3 antagonist Eliapixant, was investigated in women with predominant storage symptoms but did not meet the primary end point, the mean change from baseline over weeks 4, 8, and 12 in mean number of urgency urinary incontinence. All secondary endpoints, which typically represent storage symptoms, like micturition episodes, severe urgency episodes and nocturia episodes were also nonmeet [27, 28]. Nonetheless, one might expect that more effective and safe drugs of this class may be developed. In addition, ATP may become an interesting diagnostic or treatment follow-up biomarker for clinical use if present storage difficulties and complex measurement methods are overcome. Hence, the methodology for urine harvesting (time and temperature until storage, type of container, stabilizing solution added, time and temperature until measurement) or the development of faster, easier and cheaper methods for quantifying ATP should be addressed in the future. If these drawbacks are solved than the simple determination of ATP in the urine might allow personalized therapy with P2X3 antagonist.
This work has some limitations. Due to methodological limitations, it was not possible to characterize each animal urodynamic phenotype along the experiment. This may be achieved by performing awake cystometries and our cystometry setup requires terminal anesthesia.
In conclusion, this study revealed a significant change in the urodynamic phenotypes of animals over time following pBOO, with a predominance of DO phenotype in the early phase. These findings highlight the dynamic nature of bladder function in response to obstruction. Furthermore, our results suggest an association between the DO phenotype induced by pBOO and high urinary ATP levels, possibly linked to compensatory mechanisms developed by the bladder in response to the obstruction. This relationship may have important clinical implications, potentially providing valuable insights into the diagnosis and management of storage symptoms due to BOO in men.
SUPPLEMENTARY MATERIAL
Supplementary data can be found via https://doi.org/10.5213/inj.2346196.098.
Notes
Research Ethics
The animal procedures described in this study adhered to the in-force legislation on the protection of animals used for scientific purposes, including the European directive 2010/63/EU, the 2007/526/EC commission recommendation on guidelines for the accommodation and care of animals used for scientific purposes, and the Portuguese “Decreto-Lei nº113/2013”.
Conflict of Interest
LV and AC report no potential conflict of interest relevant to this article. FC is a consultant/researcher for Bayer.
AUTHOR CONTRIBUTION STATEMENT
· Conceptualization: FC
· Data curation: AC
· Formal analysis: LV, FC
· Methodology: LV, FC, AC
· Visualization: LV, AC
· Writing - original draft: LV, AC
· Writing - review & editing: LV, FC, AC