Adreno-Muscarinic Synergy of Contractile Responses From Human Hyperplastic Prostate
Article information
Abstract
Purpose
Adreno-muscarinic synergy, a supra-additional contractile response to simultaneous application of α-adrenoreceptor and muscarinic receptor agonists, is a feature of several lower urinary tract regions that have dual sympathetic and parasympathetic innervation. We tested the hypothesis that synergy is also a feature of prostate tissue obtained from men with benign prostatic enlargement.
Methods
Isolated tissue strips were dissected from prostate ‘chips’, collected after transurethral prostate resection procedures for in vitro experiments, to measure isometric tension at 36°C.
Results
Added separately to the superfusate, phenylephrine and carbachol generated contractions with mean pEC50 (-log10EC50) values of 5.36 and 5.58, respectively, although phenylephrine maximal responses were about six-fold greater. In the presence of carbachol, the mean phenylephrine pEC50 was significantly increased to 5.84 and maximal response increased by 28%; overall, a significant synergistic response was demonstrated. The synergistic response was reduced by muscarinic receptor antagonists, most potently by the M3-selective agent 4-DAMP (1,1-dimethyl-4-diphenylacetoxypiperidinium iodide), and less so by M2 and M1-selective inhibitors gallamine and pirenzepine, but with an overall profile indicating M3/M2 mediation of the synergistic response. The magnitude of the synergistic response was variable between prostate chips that provided isolated preparations suggesting regional heterogenicity, although their zonal origin could not be determined.
Conclusions
These experiments show that adreno-muscarinic contractile synergy is a feature of human hyperplastic prostate tissue. This has implications for the use of a combination therapy of α-blockers and anti-muscarinic agent to relieve secondary symptoms associated with benign prostatic hyperplasia, at least in men who can tolerate antimuscarinics without a risk of retention.
• HIGHLIGHTS
- Contractile responses were obtained from in vitro preparations of resected samples of human hyperplastic prostate tissue.
- An α-adrenoceptor agonist, phenylephrine, and a muscarinic receptor agonist, carbachol, given together, augmented responses compared to independent applications.
- he carbachol-induced synergy of the phenylephrine contraction was blocked most effectively by a selective M3 muscarinic receptor antagonist.
INTRODUCTION
Benign prostatic hyperplasia (BPH) is a histological diagnosis of unregulated growth of stromal, glandular and support tissue components. BPH may lead to benign prostate enlargement (BPE) and in many cases cause physical occlusion of the outflow tract —bladder outflow tract obstruction (BOO) —that can in turn be associated with the development of lower urinary tract symptoms (LUTS). However, there is no demonstrable, causal link between the extent of prostate growth, the degree of obstruction and severity of LUTS [1, 2]. For many men with BPE/BOO who present with LUTS, therapeutic treatment is often directed at either reducing prostate stromal tone with α-adrenoreceptor antagonists, or prostate size with 5,α-reductase inhibitors before resorting to surgical resection, by procedures such as transurethral resection of the prostate (TURP), if necessary. However, it would be attractive to seek additional therapeutic strategies before surgical procedures are used, in particular to more effectively regulate stromal tone.
The human prostate gland is supplied by both sympathetic and parasympathetic branches of the autonomic nervous system. Sympathetic, noradrenergic prostatic responses are mediated via α1A receptors [3] and parasympathetic muscarinic responses have been variously reported to act via M1, M2, and M3 subtypes, potentially dependent on differential distribution to stromal or epithelial cells [4-6]. Original work from our laboratory using guinea-pig bladder trigone tissue, another region of the lower urinary tract with dual functional innervation, showed that simultaneous application of α-adrenoreceptor and cholinergic receptor agonists exerted a contractile response greater than the sum of responses if either was applied independently [7-9]. This phenomenon was shown to be synergistic and attributed in part to Ca2+-sensitisation of the contractile proteins when both signalling pathways were activated. This observation has subsequently been extended to human urinary tract tissues from deeper and superficial layers of the trigone and also tissue from the prostatic urethra, with similar results [10].
Synergy of action of 2 therapeutic agents has the potential to be a more effective option for treatment of a condition, than would otherwise be expected from their individual actions. This has prompted several clinical trials to use a combination of an α-adrenoreceptor antagonist with an antimuscarinic agent to treat LUTS in patients with BPE/BOO [11-13]. Trials were restricted to patients with small postvoid residual volumes but were reported to have moderate success. However, for it to be an effective approach particularly in men with BOO/LUTS associated with BPE several basic questions also need to be addressed, and these form the basis of present study. Firstly, is there adreno-muscarinic synergy in prostate tissue from men with BPE, and secondly what is/are the muscarinic receptors responsible for mediating the synergistic response? For this reason, the study used human prostate ‘chips’ resected at time of TURP.
MATERIALS AND METHODS
Patients and Tissue Samples
Human prostate tissue was obtained from male patients (69±9 years, n=113) undergoing elective TURP for BPE. The study was approved by the local research ethics committee (R&WF 2004/209) and informed consent was attained before the procedure. Patients were scheduled for TURP and prostate ‘chips’ were irrigated out, collected at the end of the procedure and immediately placed in 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES)-buffered, nominally Ca2+ free solution. Data were collected, where possible, with respect to: urodynamic evidence of BOO (present n=69, absent n=24); presence of LUTS (present n=65, absent n=25); or acute urinary retention (present n =46, absent n =54). None of the above symptoms was present in 5 patients. International Prostate Symptom Scores (IPSS) were available from 38 patients (0–8, n=6; 9–19, n=16; ≥20, n=16).
Preparations
The edges of tissue samples were trimmed by at least 1 mm to avoid contamination by damaged or burnt regions after surgical procedures. Tissue strips, approximately 10 mm length, ≤2 mm diameter, were then dissected in HEPES-buffered, nominally Ca2+ free solution. Mucosa was dissected away, when necessary, and was identified as a smooth, pinkish layer overlaying loose connective tissue, separating it from paler, spongy glandular tissue and pale, elastic stromal tissue. Where mucosa was present, this was described as a periurethral strip. Prepared muscle strips were mounted in a horizontal superfusion trough (16-mm2 cross section), maintained at 36°C, that allowed a rapid flow rate (≈8 mL/min). Strips were tied between a fixed hook in the floor of the trough and an isometric force transducer to record tension changes.
Measurement of intracellular [Ca2+]
Intracellular Ca2+ was measured in isolated myocytes from prostate sample, by dissociation of the tissue with a collagenase solution, using the fluorochrome Fura-2 and alternate (32 Hz) illumination at 340/380 nm. The methods of myocyte isolation and signal calibration have been described in detail [14].
Protocols
All tension recordings were made with reference to a baseline value in Tyrode’s solution. All interventions were for 3 minutes or until a stable response was achieved and always followed by a return to Tyrode’s solution, i.e., cumulative concentration-response interventions were avoided. For determination of synergistic responses, preparations were exposed to one test agent (phenylephrine or carbachol) for 3 minutes followed by a combination of the two, before return to Tyrode’s solution. For experiments with muscarinic receptor antagonists, an initial Tyrode’s (12 minutes); phenylephrine (3 minutes); phenylephrine +3µM carbachol (3 minutes) cycle was established and repeated to test for stability of responses and then repeated with increasing concentrations of muscarinic receptor antagonists.
Solutions
Solutions had compositions: HEPES-buffered, nominally Ca2+ free solution (mM): NaCl, 132; HEPES, 10.0; KCl, 4.0; MgCl2, 1.0; NaH2PO4, 0.4; glucose, 6.1; Na pyruvate, 5.0; pH7.4 with 1M NaOH. Tyrode’s solution (mM): NaCl, 118; NaHCO3, 24.0; KCl, 4.0; CaCl2, 1.8; MgCl2, 1.0; NaH2PO4, 0.4; glucose, 6.1; Na pyruvate, 5.0; pH7.4 with 95%/5% O2/CO2. For a high-K+ solution, Tyrode’s solution was augmented with an additional 76 mmol/l KCl, with no osmotic correction. Stock solutions of interventions were stored (4°C) at concentrations 1,000-fold greater than the highest value used, in solvents as recommended by suppliers. All chemicals were from Sigma UK.
Data Presentation and Analyses
Data are generally presented as mean values±standard error (n= number of prostate samples), except in a few cases where data sets were obviously skewed and reported as medians (25%, 75%, interquartile range). Differences between data sets were tested by analysis of variance and post hoc parametric or nonparametric tests, as appropriate. Null hypotheses were rejected at P<0.05. Variability of measurements in a series (a, b, c…x, y, z) was estimated from calculation of the median (25%, 75%, interquartile range) of the values √(a-b)2, √(a-c)2, √(a-z)2, √(b-c)2 ...
Concentration (A)-response (T) curves were fitted to the hyperbolic relationship, equation 1:
T=(Tmax×A)/(EC50+A) (1)
where: Tmax=maximum response at high [A] and EC50=[A] required to achieve Tmax/2.
Inhibition-response curves, for variable concentrations of antagonist, C, were fitted to equation 2:
T=T1–([T2×C]/[IC50+C]) (2)
where: T1 is the response in the absence of C, and (T1–T2) is a residual response with high [C].
Assessment of synergy between 2 contractile agonists was assessed by equation 3 [15].
where: A and B refer to the 2 agonists, here phenylephrine and carbachol (effect of A>B); A2 is the [A] required to achieve a certain amount of force alone (say 50% of maximum tension, Tmax) and A1 is [A] in the presence of B. AEC50 (BEC50) are concentrations required to achieve 50% Tmax. The equation therefore calculates the concentration of one agonist required to generate a certain amount of force in the presence of a second, independent agonist, deviation from this calculation presumes synergy (for a greater response) or antagonism (for a smaller response). EC50 values are also shown as pEC50 (-log10EC50) values. Estimation of the inhibitor constant, Ki, for a carbachol (B) receptor antagonist, from the IC50 value used a Cheng-Prusoff formalism [16]:
Ki=IC50/(1+([B]/BEC50)), where [B]=3µM (4) (4)
RESULTS
Individual Responses to Phenylephrine and Carbachol
Phenylephrine generated a contractile response in 82% (n=278) preparations that generally consisted of a contracture and superimposed transients; for this study steady-state responses were quantitatively analysed (Fig. 1A). Contractile responses were completely abolished by 1µM phentolamine. Concentration-response curves (1–30µM) yielded a maximum tension, Tmax, of 2.17±0.34 mN/mm2 and a pEC50 of 5.36±0.09 (mean EC50=4.37µM, n=29: Fig. 1B). In comparison maximal contractile responses to depolarisation with 80 mM high-K+ solution was 1.01±0.94 mN/mm2 (n=7).
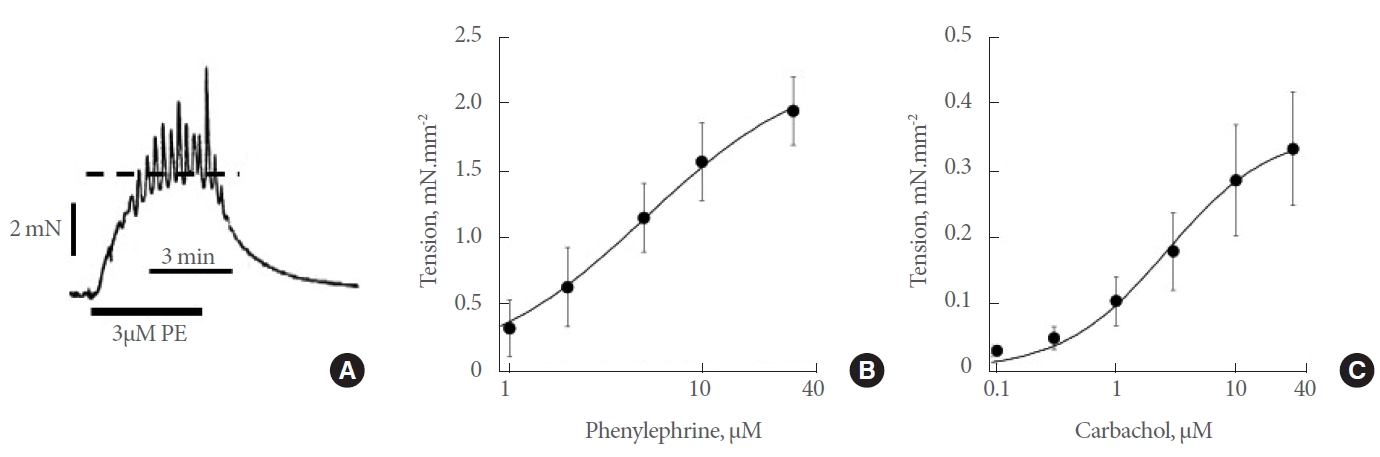
Effects of contractile agonists on human prostate in vitro preparations. (A) Sample response to 3μM phenylephrine (PE). The dotted line shows the peak of the steady-state response. (B) Concentration-response curve (1–30μM, n=29) to PE. (C) Concentrationresponse curve (0.1–30μM, n=9) to carbachol. Curve fits to parts B and C with equation 1; data mean±standard deviation values.
Carbachol generated much smaller contractures and only in 63% of 191 preparations. Concentration-response curves (0.1–30µM) yielded a maximum tension of 0.36±0.09 mN/mm2 and a pEC50 of 5.58±0.07 (mean EC50=2.63µM, n=9; Fig. 1C). Test concentrations of 3µM for phenylephrine and carbachol were used as required in subsequent experiments when single agonist concentrations were required.
Combined Responses From Phenylephrine and Carbachol
Carbachol (3µM) in the continued presence of phenylephrine (3µM) augmented the final response in 67% of preparations responding to phenylephrine alone (115 of 171 preparations); Fig. 2A where the response to carbachol alone was very small. The range of augmentation varied greatly from 10%–400% of the effect of phenylephrine alone, and these values were not normally distributed (Fig. 2B), with a median increase of 96% (52%, 144%, interquartiles) of control.

Synergistic actions of phenylephrine (PE) and carbachol on in vitro human prostate contractions. (A) Sample response to 3μM PE, with carbachol (carb) applied after 5 minutes. Dotted lines show peak steady-state responses before and after carbachol. (B) Histogram of the percentage increase of a 3μM PE contraction induced by 3μM carbachol. (C) Concentration-response curves (1–30μM, n=12) to PE in the absence (upper curve) and presence of 3μM carbachol. Curve fits to equation 1, mean±standard deviation (SD) values. (D) Calculated concentration-response curve (0.1–30μM) to PE in the absence (solid line) and presence (interrupted line) of carbachol, as well as a curve assuming independent actions of the 2 agents (equation 3, dotted line). The EC50 values (±SD) are shown for the 2 PE concentration-response curves.
Reasons for the variability of the synergistic response were explored as: (1) relevant clinical descriptors of patients from whom chips were obtained; (2) variation between experimental preparations from prostate chips. There were no significant associations between the presence or magnitude of the synergistic response to carbachol and the presence of BOO, LUTS or acute retention; prostate size (stratified as small, moderate or large); nor with the IPSS score of patients donating prostate chips. Neither were there associations with a history of either prostate malignancy nor use of α-adrenoreceptor antagonists prior to surgery.
The median variability of the magnitude of synergistic responses to carbachol was estimated (see Methods). Repeated application of the protocol in Fig. 2A, showed little variability (8% [3%–19%] from the first application (22 comparisons from n =14 strips). When it was possible to prepare several strips from one chip, variability of the extent of synergy was not significantly different from infrastrip variability (21% [9%, 36%, interquartiles]; 51 comparisons from n =20 strips, P >0.05). However, preparations from different chips of the same patient demonstrated significantly more variability (40% [21%, 69%, interquartiles]; 120 comparisons from n=48 patients, P<0.05). This interchip variability was not significantly different from the first response from each patient of the population (46% [21%, 80%, interquartiles]; 2,346 comparisons from n=70 patients, P>0.05). Thus, the greatest change of variability was between comparing infrachip and interchip variance from the same patient and is consistent with there being regional differences of carbachol-dependent synergy within the prostate.
Augmentation of phenylephrine responses by 3µM carbachol was measured over a range of concentrations (1–30µM) in a separate series of experiments. In comparison to the presence of phenylephrine alone, the concentration-response curve showed a significant increase of efficacy (2.69±0.28 mN/mm2 vs. 2.10±0.15 mN/mm2, P<0.05, n=12) and a significant increase of potency from a greater pEC50 value (5.84 ±0.09 vs. 5.40 ±0.15, P<0.05) (Fig. 2C).
A complementary estimation of synergy between responses to contractile agonists may be gained through the generation of a mean phenylephrine concentration-response curve in the presence of carbachol, using equation 3 (see Methods), assuming the 2 agonists act independently, with Tmax and EC50 values for phenylephrine and carbachol as given above, Fig. 2D. Because the effect if carbachol alone is very small, this curve (equation 3) is similar to that of phenylephrine alone. The experimental concentration-response phenylephrine curve with carbachol lies above and to the left of the fitted curve, suggesting synergy of action between the 2 contractile agonists. The pEC50 values have been included on the 2 concentration-response curves to phenylephrine to emphasise the significance of the increase of potency in the presence of carbachol.
Intracellular Ca2+ was measured in 5 viable myocytes where 10µM phenylephrine generated a transient rise of intracellular [Ca2+], increasing from a baseline of 234±34 to 347±80nM. The increase was not significantly different in the additional presence of 10µM carbachol (178±44 to 251±80nM, P>0.05). Therefore, augmentation of the phenylephrine contraction by carbachol was not mirrored by a greater magnitude of the intracellular [Ca2+].
Phenylephrine/Cholinergic Synergy and Muscarinic Receptor Antagonists
The role of different muscarinic receptors to elicit carbachol-induced synergy was tested by measuring the ability of 3µM carbachol to augment 3µM phenylephrine (3µM) in the absence and presence of selective muscarinic receptor antagonists: 1,1-dimethyl-4-diphenylacetoxypiperidinium iodide (4-DAMP) pirenzepine or gallamine.
4-DAMP data
The initial augmentation by 3µM carbachol of the contracture elicited by 3µM phenylephrine was by 151.0% ±7.7% (n =8, Fig. 3B). 4-DAMP (10nM–1µM) reduced the augmentation in a dose-dependent manner and was completely abolished at 1µM 4-DAMP (Fig. 3A). At the highest concentration (1µM) the residual augmentation was 2.6%±5.9% (Fig. 3B). The concentration-response curve had a pIC50 of 7.82 ±0.50 (n =8, mean IC50=15.3nM, with a calculated pKb value of 8.15) and had a significantly (P<0.05) greater value than that of pirenzepine or gallamine.

Inhibition of the synergistic actions of phenylephrine (PE) and carbachol by selective muscarinic receptors antagonists. (A) Sample response to 3μM PE, alone or in combination with carbachol; selective muscarinic receptor antagonists (e.g., 1,1-dimethyl-4-diphenylacetoxypiperidinium iodide [4-DAMP]) added as shown. (B) Inhibition- response curve of 4-DAMP (1–1,000nM, M3-selective inhibitor, n=8) – the square shows the response in the absence of the inhibitor. (C) Inhibition-response curve of pirenzipine (0.1–10μM, M1-selective inhibitor, n=5). (D) Inhibition-response curve of gallamine (0.1–10μM, M2-selective inhibitor, n=5). Curves fitted to equation 2, data mean±standard error of the mean.
Pirenzipine data
The initial augmentation of the phenylephrine contracture was by 91.5%±1.6% (n=5, Fig. 3C). Pirenzipine (0.1–10µM) also reduced the augmentation in a dose-dependent manner, but did not abolish it at the highest concentrations, leaving a significant residual action of carbachol of 13.4%±1.2% (n=5, P<0.05); the pIC50 of 6.41±0.62 (n=5, mean IC50=0.39µM, calculated pKb=6.74).
Gallamine data
The initial augmentation of the phenylephrine contracture was by 123.6%±10.2% (n=5, Fig. 3D). Data were similar to those with pirenzepine, with a dose-dependent (0.1–10µM) reduction of augmentation and a significant residual at high concentrations of 26.5%±1.9% (n=5, P<0.05). The pIC50 of 6.52±0.58 (n=5, mean IC50=0.30µM, calculated pKb=6.85) was not significantly different (P>0.05) from that for pirenzipine.
DISCUSSION
Responses to Phenylephrine and Carbachol
This study has shown the feasibility of obtaining functional in vitro preparations of enlarged human prostate tissue from resected ‘chips’ following TURP procedures. Other studies on the contractile properties of human prostate have used samples obtained after radical prostatectomy for prostate cancer, but distant to tumour sites and generally in the periurethral zone. Similar phenylephrine EC50 values (in the range 1–10µM) to those recorded in this study have been obtained, as well as similar magnitude responses as a proportion of high-K contractures [17-19]. This implies that the contractile potency and efficacy of an α-adrenoreceptor agonist such as phenylephrine are similar in enlarged and relatively normal prostate tissue. Contractile responses to cholinergic receptor agonists were recorded in about two-thirds of preparations but were much smaller than those to phenylephrine; the EC50 value (2.6µM) was similar to that recorded for canine prostate (EC50=5.9µM [20]). Variable carbachol responses are reflected in other studies with human prostate, some recording small contractions [19] whilst others none [21, 22]; a contributor to this variability is considered below.
Synergy of Phenylephrine and Carbachol Responses
The most striking feature was augmentation of phenylephrine responses by carbachol, when primary carbachol responses alone were small. This has been observed in other lower urinary tract regions also with a dual sympathetic/parasympathetic innervation (e.g., trigone and urethra), suggesting this is a normal feature under such circumstances [7, 9, 10]. With the trigone, Ca2+ sensitisation of the contractile proteins with a dual adreno-muscarinic input was proposed as a mechanism to augment force on addition of a second transmitter in the presence of the first. This is because contractile synergy was not mirrored by intracellular [Ca2+], [Ca2+]i, changes in isolated cells, i.e., addition of a second transmitter did not further raise [Ca2+]i. In this study, measurement of [Ca2+]i was possible in 5 viable, isolated prostate stromal myocytes which also showed that the increase on intracellular [Ca2+]i with phenylephrine was not significantly different in the absence or presence of carbachol. We therefore hypothesise that a similar cellular mechanism for adreno-muscarinic synergy as shown in trigone is also present in human prostate stromal cells.
Despite the poor contractile response to carbachol alone, the density of muscarinic receptors in human prostate is greater than that of α-adrenoreceptors [19], it would therefore be valuable to identify the muscarinic receptor subtype involved in the synergistic response. The majority are M1 receptor subtypes, located in glandular epithelium, and the much smaller population of M2 receptors are in stromal cells [4, 5, 23, 24]. Less is known about the distribution of M3 receptors, but by analogy with detrusor smooth muscle may colocate in the stroma and in animal studies, M3 receptors underlie contractile responses to acetylcholine [25].
The rank order of potency for muscarinic antagonists to reduce the synergistic response was 4-DAMP> gallamine≈pirenzepine and furthermore only 4-DAMP completely abolished carbacholinduced synergy. 4-DAMP shows a selectivity towards M3 receptors with an estimated pKb >9.3 in cloned receptors [26] and detrusor smooth muscle contractile assays [27, 28]. These values are significantly greater that the pKb (=8.15) for abolition of the synergistic response by 4-DAMP. It is therefore possible that M2 receptors also contribute, as the selectivity for M3 over M2 by 4-DAMP is only some 30-fold [26], and also the pKb for the M2-selective antagonist gallamine to reduce the response (pKb=6.85) is similar to the value for binding to M2 receptors [29]. M1 receptors are unlikely to be involved as the pKb for the M1-selective agent pirenzepine (=8.35 [29]) was much greater than the value obtained in this study (pKb=6.74).
Variability of in Adreno-Muscarinic Synergy in the Prostate
One limitation working with human tissue samples is the presence of confounding factors that impact on the reproducibility of data; exemplified by the wide variability of synergistic responses to phenylephrine in the presence of carbachol. Any effect was observed in only 67% of preparations and in these the increase varied widely (Fig. 2B). No relationship was seen with several clinical descriptors (Results) such as: presence or absence of BOO; LUTS; urinary retention; prostate size; IPSS score; history of prostate malignancy; or use of α-blocker. However, a major source of variability was between different prostate chips from which experimental preparations were dissected, but not within a given chip and suggests variability within the prostate itself. This was supported by the fact that a synergistic response was observed in 82.1% of chips with adherent mucosa (removed before muscle strips were prepared but suggests a periurethral origin), but in only 57.9% where there was none. Any regional variability might contribute to the different responses to carbachol reported here and in the literature [19-22]. This does not exclude clinical variation affecting the synergistic response, but regional variation in the prostate itself may be of greater significance.
In conclusion, these data show it is feasible to use resected prostate strips to investigate contractile properties of human prostate. Of interest is a synergistic contractile relationship between α-adrenoreceptor and muscarinic receptor agonists, the latter mediated by M2/M3 subtypes. In principle, this gives credibility to the use of α-blocker and antimuscarinic agent combination therapy to better relieve LUTS. Such combination therapy is supported by evidence [30-32], although its effectiveness remains debated [33] and use is limited by concern regarding urinary retention in men with elevated postvoid residual (presumably due to effects of antimuscarinics on bladder contractile function) [13]. Further investigations to determine if the selectivity profile of muscarinic receptors might better target symptoms, whilst maintaining safety.
Notes
Grant/Fund Support
The study was funded by The Prostate Research Campaign, UK.
Research Ethics
The study was performed according to the Helsinki Declaration and approved by the local research ethics committee (Redbridge and Waltham Forest [London] – reference: R&WF 2004/209). Informed consent was attained from all patients for tissue retrieval.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTION STATEMENT
· Conceptualization: BTBJ, CHF
· Data curation: BTBJ, CHF
· Formal analysis: BTBJ, CHF
· Funding acquisition: CHF
· Methodology: BTBJ, CHF
· Project administration: CHF
· Visualization: BTBJ, CHF
· Writing – original draft: CHF
· Writing – review & editing: BTBJ, BC, CHF
