|
 |


- Search
| Int Neurourol J > Volume 28(1); 2024 > Article |
|
ABSTRACT
Urinary tract infections (UTIs) are among the most common bacterial infections worldwide and are particularly prevalent in women. Recurrent UTIs significantly diminish quality of life due to their symptoms and frequent relapses. Patients often experience immediate relapse following slightly strenuous activities or intense psychological stress. In this review, we explore why infections persist despite the advent of various treatments and suggest strategies to manage recurrent cystitis by targeting the mechanisms of adhesion and infection. Vitamin D levels and the expression of neutrophil gelatinase-associated lipocalin are linked to the recurrence of UTIs. During a UTI, bacteria employ adhesins to invade the urinary tract, adhere to urothelial cells, and then penetrate these cells, where they rapidly multiply to establish intracellular bacterial communities. Bacteria can also form quiescent intracellular reservoirs that escape immune responses and antibiotic treatments, leading to recurrence under certain conditions. The surface proteins of bacteria and D-mannose are crucial in the adhesion of bacteria to the urothelium. Understanding these processes provides valuable insights into potential therapeutic approaches that focus on preventing bacterial attachment and cluster formation. By disrupting the ability of bacteria to adhere to and form clusters on cells, we can better manage recurrent UTIs and improve patient outcomes.
Urinary tract infections (UTIs) are among the most common bacterial infections worldwide and are more likely to occur in women than in men. By the age of 24, at least one-third of women will have experienced a UTI, and nearly half of all women will have had one or more UTI episodes in their lifetime [1]. UTIs typically manifest with urinary dysfunction symptoms such as dysuria, increased urinary frequency, and urgency, all of which can adversely affect quality of life. Recurrent UTIs (rUTIs) are characterized by the reoccurrence of an acute bacterial infection caused by the same pathogen. An rUTI is defined as experiencing 2 or more episodes within 6 months or 3 or more episodes within a year [2,3]. rUTIs are categorized into relapses, where symptoms return due to the same organism not being completely eradicated after appropriate treatment, and reinfections, where symptoms are caused by a new microbial infection following the complete clearance of the original microbe. However, clinically distinguishing between these 2 scenarios is challenging [4].
Uncomplicated UTIs refer to those that occur in the urinary tract of a healthy host without any structural or functional abnormalities. Recurrent uncomplicated UTIs, often caused by the same bacteria, are most commonly seen in young women. Currently, urine culture is considered the gold standard for diagnosing recurrent uncomplicated UTIs, with additional clinical judgment based on positive results from dipstick tests and microscopy. Typically, the clinical diagnosis of a UTI episode is made based on symptoms such as dysuria, frequency, urgency, hematuria, and back pain. When symptoms are present, treatment is initiated, and a UTI is confirmed by culture 2 weeks posttreatment. If the infection persists, further treatment is administered. For diagnosing UTIs in patients, a urine culture is indicated when the number of colony-forming units per milliliter (CFU/mL) of urine is 100 or greater. In cases where anatomical abnormalities are suspected, various diagnostic techniques, including imaging studies, are employed to manage recurrent UTIs [5,6].
The concept of defining a UTI based on urine bacterial counts was first introduced by Kass in 1957 [7]. Traditionally, a UTI has been characterized by the presence of typical acute symptoms and a urine culture with 1×105 CFU/mL or more. However, this definition may prevent the diagnosis of cystitis in many patients, particularly those with infections that present with lower bacterial counts. In 1982, Stamm et al. suggested that a lower threshold of 1×102 CFU/mL of urine should be considered appropriate for the diagnosis of simple and recurrent UTIs [8]. Furthermore, when standard test results are negative, alternative diagnostic methods such as next-generation sequencing, deep metagenomic sequencing, pooled antibiotic susceptibility testing, and expanded quantitative urine culture may be utilized [9].
rUTIs pose serious problems for clinical management and can severely impact patient quality of life. Although most UTIs are attributed to extracellular bacteria, emerging research has highlighted the significance of intracellular bacterial communities (IBCs) in the persistence of rUTIs. Bacteria gain entry to the bladder via the urethra and adhere to the luminal surface of bladder epithelial cells by expressing type 1 pili. Once these pathogens invade the bladder, they establish biofilm-like IBCs that are not completely eradicated, thus contributing to recurrent episodes. The bacteria that have attached themselves to the bladder wall then invade the urothelium to form IBCs. An IBC represents a transient bacterial state that persists until the bacteria disperse and are released from the host cells. Bacteria capable of forming IBCs can evade phagocytosis by neutrophils and can cause reinfection by infiltrating deeper into the urothelium. These IBCs can survive within host cells, shielded from the effects of antibiotics and immune responses, and they possess distinctive characteristics that facilitate the recurrence of infection. A deeper understanding of IBC formation dynamics and their role in rUTIs is essential for advancing our comprehension of UTI pathogenesis [10-12].
The identification and characterization of IBCs are critical for tackling the complexities of rUTIs. Recent advances in microscopy, molecular biology, and diagnostic imaging have equipped researchers with sophisticated tools that allow for the visualization and detailed study of IBCs in unprecedented detail [13].
We reviewed existing studies and compiled the latest research findings on the role of IBCs in rUTIs. Additionally, we evaluated the potential of detecting IBCs as a diagnostic marker. The insights gained from this comprehensive review suggest innovative strategies for the more accurate and timely identification of rUTI cases. Ultimately, a deeper understanding of IBCs in the context of rUTIs could pave the way for targeted therapeutic interventions and preventive measures, thereby improving the clinical management of this challenging condition.
In women, the urethra is situated closer to the anus than in men, and the microbiome is in closer proximity to the urethra. This anatomical arrangement facilitates the transfer of pathogens, such as uropathogenic Escherichia coli (UPEC), from the fecal reservoir to the bladder and renal pelvis. The female urethra is also shorter than its male counterpart, which allows pathogens to reach the bladder more easily [14,15]. Women are at a higher risk of rUTIs due to a variety of factors, including sexual activity, changes associated with urogenital aging, pelvic organ prolapse, menopause, and pregnancy [16].
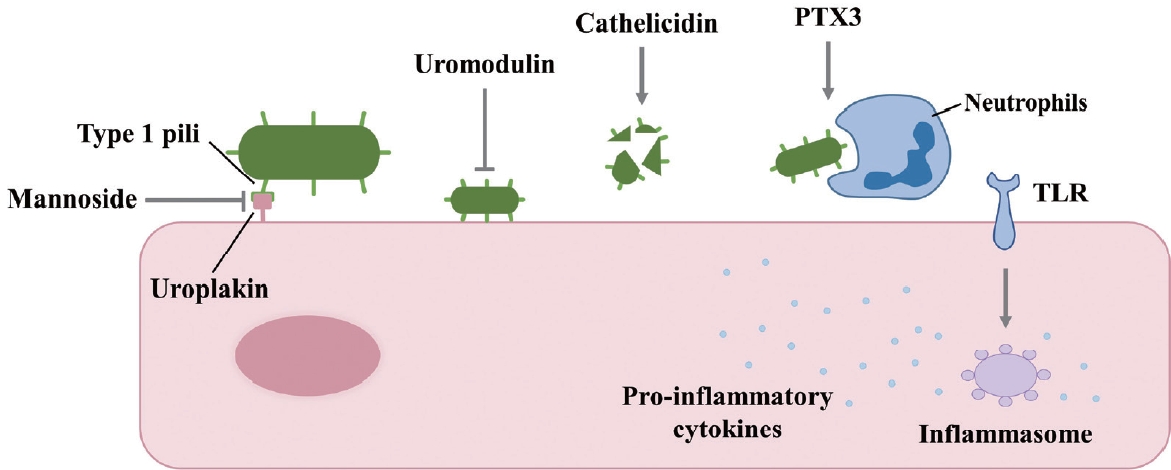
E. coli is the predominant pathogen found in the urine samples of patients with UTIs [17]. This is attributed to the specific adhesin protein PapG, which is located at the distal tip of the P pilus of UPEC and readily binds to kidney epithelial cells. Additionally, type 1 pili can attach to uroplakin on the surface of urothelial cells in the bladder [18]. Understanding the mechanism of UPEC persistence is crucial for managing rUTIs. In a mouse model, F17-like and type 1 pili were demonstrated to facilitate intestinal colonization and adherence to epithelial cells along the colonic crypts. Furthermore, the mannoside M4284, a potent inhibitor of FimH, significantly reduced the incidence of rUTIs [19]. When UPEC initiates an infection, the urinary tract’s immune response is also involved. Uromodulin, produced by the urothelium, impedes UPEC adherence. Cathelicidin disrupts bacterial membranes, leading to lysis. Pentraxin 3 hinders uropathogens and enhances their phagocytosis. The inflammasome, activated by Toll-like receptors (TLRs), produces proinflammatory cytokines [18]. UPEC is a bacterial species frequently implicated in UTIs. Its ability to form biofilm-like structures, known as IBCs, is facilitated by a polysaccharide matrix that is encased within a uroplakin covering [20] (Fig. 1).
rUTIs are closely associated with antibiotic resistance. It is crucial to understand that the overuse or misuse of antibiotics can contribute to the development of antibiotic resistance, making it difficult to treat infections. All isolates of E. coli have been reported to be resistant to ampicillin, cefazolin, ceftazidime, ceftriaxone, and cefepime. These antibiotics, which belong to different classes, exhibit widespread resistance, posing a significant challenge for the effective treatment of UTIs [21]. High levels of antibiotic resistance have also been observed in urine cultures from various laboratories, including resistance to trimethoprim-sulfamethoxazole (50%), ciprofloxacin (38.2%), levofloxacin (36.7%), and norfloxacin (36.5%) [22]. In patients with rUTIs, the increased global use of antibiotics, prolonged use of prophylactic antibiotics, and the use of multiple different antibiotics can lead to the development of further resistance.
IBCs and quiescent intracellular reservoirs (QIRs) are mechanisms that contribute to the recurrence of UTIs and the evolution of antibiotic resistance. The P pili and type 1 pili of UPEC can attach to uroplakin 1a on the urothelial tissue and subsequently invade cyclic AMP-dependent Rab27b/CD63-positive vesicles [23]. These endocytic mechanisms lead to the rearrangement of the host cytoskeleton. Upon attachment to the plasma membrane, Rho GTPases, tyrosine kinases, phosphoinositide-3-kinase, and focal adhesion kinases are activated [24]. Murine cystitis models have shown that the innate immune response is mediated by TLR4 in response to the activation of nuclear factor kappa B and the subsequent secretion of cytokines [25]. Once UPEC is internalized by the host cell, it rapidly replicates and forms a cytosolic developmental stage known as an IBC, which exhibits antibiotic resistance. Autotransporter antigen 43 is a protein present in certain E. coli strains that facilitates cell aggregation and biofilm formation [26].
UPEC in IBCs may be released from the host cell, reinitiating the cycle of infection that leads to rUTIs. Alternatively, UPEC can establish a QIR by becoming encapsulated in LAMP-1 (lysosome-associated membrane protein-1)-positive endosomes [12]. These dormant bacteria neither replicate nor provoke an immune response [27]. QIRs have been identified in asymptomatic patients with rUTIs [28].
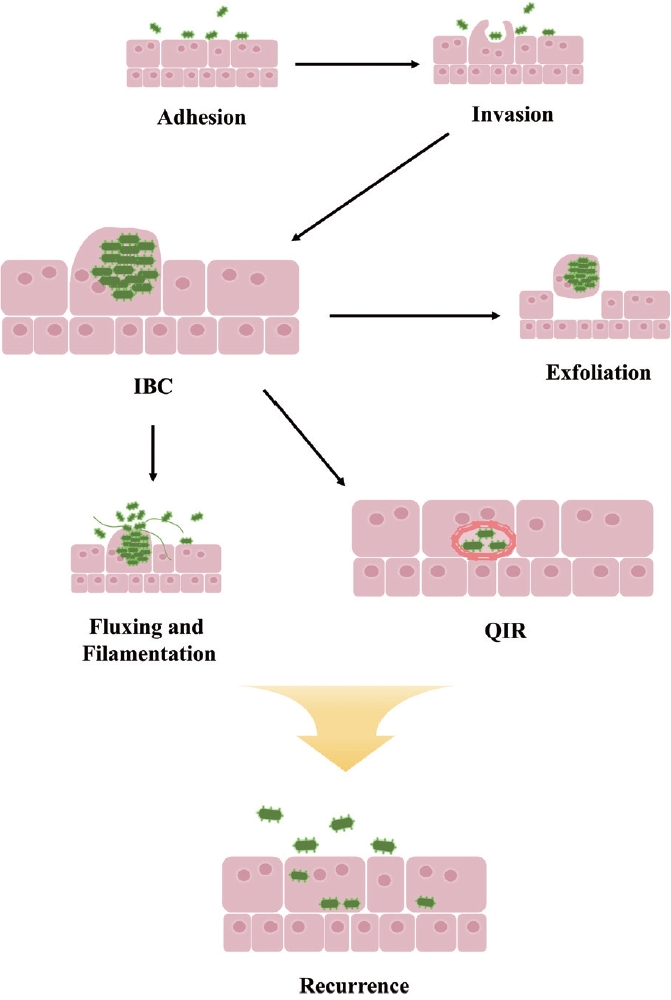
In summary, bacteria can invade the urinary tract by utilizing various adhesins to adhere to urothelial cells. Once attached, they can invade these cells and rapidly replicate to form IBCs. These bacterial clusters may either be exfoliated and expelled in the urine or undergo filamentation to evade engulfment by neutrophils and facilitate reinvasion of the urothelium. Alternatively, bacteria may form QIRs to survive immune defenses or antibiotic treatment, leading to recurrence in certain environments (Fig. 2).
Intracellular UPEC undergoes multiple stages of the developmental cycle—invasion, IBC formation, IBC maturation, and QIR formation—which contribute to rUTIs [24]. Following invasion, UPEC rapidly replicates for up to 8 hours, forming a rod shape. This significant increase in the number of infectious bacteria within the bladder facilitates UPEC spread and persistence. In the intermediate stage, these rod-shaped bacteria become densely packed, forming biofilm-like IBCs that are resistant to antibiotics. The transition from IBCs to QIRs represents a mechanism by which some bacteria establish a persistent infection. The ability to enter a dormant state enables bacteria to withstand adverse conditions, such as antibiotic exposure or an activated immune response. Since bacteria in a quiescent state neither actively replicate nor provoke inflammation, they can evade host defenses [24].
In 2021, Sharma et al. [29] reported the dynamic persistence of IBCs based on a human bladder-chip model of UTI. They created a bladder-chip infection model through the coculture of endothelial cells with an epithelial cell line. A significant observation was that antibiotic treatment did not guarantee the complete eradication of bacteria within IBCs; in some cases, bacterial elimination was either delayed or did not occur at all. This study indicates that IBCs can persist and potentially lead to the reseeding of infection sites, even after antibiotics have been administered. Adherence to prescribed antibiotic regimens is particularly critical in the context of IBCs, as these communities can quickly reestablish infection sites in the bladder when doses are missed. Therefore, ensuring strict compliance with antibiotic therapy is essential for effectively tackling bacterial infections and preventing their recurrence or dissemination [29].
rUTIs require repeated antibiotic treatment and preventive therapy. However, prolonged antibiotic use can lead to increased resistance, potentially resulting in UTI recurrence due to treatment failure. To prevent recurrent cystitis, it is crucial to anticipate a patient’s resistance and susceptibility patterns. A retrospective study that analyzed gram-negative uropathogens isolated from the urine of patients with recurrent, uncomplicated cystitis found significant resistance to earlier-generation cephalosporins, trimethoprim-sulfamethoxazole, and fluoroquinolones. However, these pathogens remained susceptible to nitrofurantoin. Consequently, prior urine culture can be instrumental in optimizing empirical antibiotic prescriptions for patients with recurrent cystitis [30].
Additionally, rUTIs can be influenced by a range of patientspecific factors; thus, employing a machine learning model may enhance the prediction of rUTIs. A well-validated machine learning model has the potential to significantly improve the accuracy of forecasting rUTIs, particularly those caused by a single uropathogen, such as E. coli. The dataset utilized for developing this predictive model encompasses both patient and bacterial characteristics. Patient factors comprise medical history, clinical symptoms, and results from laboratory tests. Bacterial factors, on the other hand, include antibiotic resistance patterns, bacterial phylogeny, and the presence of virulence genes. By integrating these factors, the model can assist physicians in implementing preventative strategies for rUTIs [31].
Several studies have revealed that serum biomarkers may be instrumental in predicting and preventing rUTIs. Serum cytokine analysis in mice with acute bacterial cystitis has shown that the expression of cyclooxygenase-2 during an acute UTI is significant [32]. Furthermore, premenopausal women with rUTIs exhibit notably lower average serum vitamin D levels than controls, implying an association between rUTIs and vitamin D deficiency [33].
Research has been conducted to predict rUTIs through the analysis of urinary biomarkers. Neutrophil gelatinase-associated lipocalin (NGAL) is expressed in damaged kidneys, the liver, and epithelial cells within inflammatory and infectious environments. It is upregulated in the urothelium and kidneys of patients with UTIs. Furthermore, some patients with rUTIs exhibit a relative local deficiency in urinary NGAL production, rendering them more susceptible to UTIs [34-36].
Bacteria can enter the bladder via the urethra and proliferate by adhering to the urothelium or to implanted foreign bodies, which are shielded by a biofilm [37]. Additionally, they can infiltrate the urothelium and establish IBCs, resulting in chronic infections. These bacterial aggregates can complicate detection and treatment. Therefore, it is important to identify and eliminate potential IBCs.
Biofilm formation by bacteria offers a defense against assaults from the immune system and antifungal drugs by secreting an extracellular matrix. Mitogen-activated protein (MAP) kinases and phosphatases are crucial in the signaling pathways that control cell wall composition, extracellular matrix production, adhesion, and biofilm formation in Aspergillus fumigatus. The absence of MAP kinases and phosphatases leads to reduced attachment and biofilm formation [38].
In Streptococcus parasanguinis FW213, the cell surface protein BapA1 is essential for bacterial adhesion and biofilm formation due to its role in intercellular interactions. Mutating BapA1 reduced dental biofilm development in an in vitro tooth model and diminished bacterial autoaggregation [39].
Staphylococcus epidermidis strains HB and K28 strongly adhere to immobilized fibrinogen, facilitated by the expression of the surface protein Fbe. Mutations in the Fbe gene can significantly impact the attachment capability of these strains [40].
D-mannose inhibits the adhesion and invasion of E. coli in patients with rUTIs, preventing the formation of quiescent bacterial reservoirs [41]. Bacteria that enter the urinary tract can cause infections. The urinary mucosa is coated with proteins that impede bacterial attachment. D-mannose, a monosaccharide, is readily absorbed and excreted through the urinary tract. It is structurally similar to the binding sites of glycoprotein receptors on urothelial cells, allowing it to competitively inhibit the attachment of E. coli to these cells. At a concentration of approximately 4 mg/mL, D-mannose can almost completely prevent the attachment of type 1 bacterial fimbriae to urothelial cells. Furthermore, D-mannose can inhibit bacterial invasion of urothelial cells and biofilm formation at low concentrations. Despite a significant loss of dosage before reaching the urine, D-mannose facilitates bacterial elimination and helps in preventing infection. Nonantibiotic approaches, such as D-mannose, are increasingly being considered for UTI treatment due to the side effects and antibiotic resistance associated with antibiotic therapy.
Why does cystitis recur immediately after the onset of fatigue? Patients with recurrent cystitis often report an immediate return of symptoms when they engage in even mildly strenuous activities or experience significant psychological stress. They seek an explanation. How should urologists respond? This review presents evidence that may elucidate the reasons for UTI recurrence. Most E. coli internalized by urothelial cells are rapidly expelled through exocytosis. However, some bacteria evade this defense and initiate a cytosolic phase known as IBC formation. As IBCs develop, often near the host cell nuclei, bacteria at the periphery of the community revert to a rod shape and disperse, while others transform into long filaments that extend from the infected cell. E. coli employs multiple strategies to alter the host’s immune response to infection. To establish and maintain colonization, uropathogens must overcome the innate and adaptive immune defenses of the host. This process involves the upregulation of various cytokines and chemokines. Changes in the components of the host immune system can affect susceptibility to infection and its outcomes. E. coli can also infect the basal epithelium, forming a QIR. In this state, E. coli can persist for months, enclosed in a membrane and exhibiting antibiotic resistance, even without bacterial replication. This reservoir is a key factor in the recurrence of UTIs, particularly in women [23,42].
Given that much of our understanding of the lifecycle of E. coli in UTIs comes from studies in murine models, there is a need for further research to fully comprehend this host-pathogen interaction in humans.
REFERENCES
1. Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Dis Mon 2003;49:53-70. PMID: 12601337


2. Gopal M, Northington G, Arya L.. Clinical symptoms predictive of recurrent urinary tract infections. Am J Obstet Gynecol 2007;197:74.e1-4. PMID: 17618765


3. Albert X, Huertas I, Pereiró II, Sanfélix J, Gosalbes V, Perrota C. Antibiotics for preventing recurrent urinary tract infection in non‐pregnant women. Cochrane Database Syst Rev 2004;2004:CD001209. PMID: 15266443



4. Meštrović T, Matijašić M, Perić M, Čipčić Paljetak H, Barešić A, Verbanac D. The role of gut, vaginal, and urinary microbiome in urinary tract infections: from bench to bedside. Diagnostics (Basel) 2020;11:7. PMID: 33375202



5. Dason S, Dason JT, Kapoor A. Guidelines for the diagnosis and management of recurrent urinary tract infection in women. Can Urol Assoc J 2011;5:316-22. PMID: 22031610



6. Choi H, Bae JH. How do you diagnose recurrent urinary tract infections and confirm the diagnosis? Urogenit Tract Infect 2016;11:93-6. 

7. Kass EH. Bacteriuria and the diagnosis of infections of the urinary tract: with observations on the use of methionine as a urinary antiseptic. AMA Arch Intern Med 1957;100:709-14. 
8. Stamm WE, Counts GW, Running KR, Fihn S, Turck M, Holmes KK. Diagnosis of coliform infection in acutely dysuric women. N Engl J Med 1982;307:463-8. 
9. Huang L, Li X, Zheng B, Li P, Wei D, Huang C, et al. Differential urinary microbiota composition between women with and without recurrent urinary tract infection. Front Microbiol 2022;13:888681. PMID: 35722326



10. Rosen DA, Hooton TM, Stamm WE, Humphrey PA, Hultgren SJ. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med 2007;4:e329. PMID: 18092884



11. Anderson GG, Palermo JJ, Schilling JD, Roth R, Heuser J, Hultgren SJ. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 2003;301:105-7. PMID: 12843396


12. Mysorekar IU, Hultgren SJ. Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proc Natl Acad Sci U S A 2006;103:14170-5. PMID: 16968784



13. Zhao A, Sun J, Liu Y. Understanding bacterial biofilms: from definition to treatment strategies. Front Cell Infect Microbiol 2023;13:1137947. PMID: 37091673



14. Magistro G, Marcon J, Schubert S, Gratzke C, Stief CG. Pathogenese der harnwegsinfektion: ein update [Pathogenesis of urinary tract infections: an update]. Urologe A 2017;56:720-7 (German). PMID: 28455576

15. Delcaru C, Alexandru I, Podgoreanu P, Grosu M, Stavropoulos E, Chifiriuc MC, et al. Microbial biofilms in urinary tract infections and prostatitis: etiology, pathogenicity, and combating strategies. Pathogens 2016;5:65. PMID: 27916925



16. Kim A, Ahn J, Choi WS, Park HK, Kim S, Paick SH, et al. What is the cause of recurrent urinary tract infection? Contemporary microscopic concepts of pathophysiology. Int Neurourol J 2021;25:192-201. PMID: 34044483




17. Jhora ST, Paul S. Urinary tract infections caused by Staphylococcus saprophyticus and their antimicrobial sensitivity pattern in young adult women. Bangladesh J Med Microbiol 2011;5:21-5. 

18. Ortega Martell JA. Immunology of urinary tract infections. GMS Infect Dis 2020;8:Doc21. PMID: 32821646


19. Spaulding CN, Klein RD, Ruer S, Kau AL, Schreiber HL, Cusumano ZT, et al. Selective depletion of uropathogenic E. coli from the gut by a FimH antagonist. Nature 2017;546:528-32. 

20. Sihra N, Goodman A, Zakri R, Sahai A, Malde S. Nonantibiotic prevention and management of recurrent urinary tract infection. Nat Rev Urol 2018;15:750-76. PMID: 30361493



21. Abed BK, Authman SH, Yassin KH. In vitro antibacterial activity of iron oxide nanoparticles against Escherichia coli isolated from UTI. Int Res J Nat App Sci 2016;3:58-67.
22. Zúniga-Moya JC, Bejarano-Cáceres S, Valenzuela-Cervantes H, Gough-Coto S, Castro-Mejía A, Chinchilla-López C, et al. Antibiotic sensitivity profile of bacteria in urinary tract infections. Acta Méd Costarric 2016;58:146-54.
23. Bishop BL, Duncan MJ, Song J, Li G, Zaas D, Abraham SN. Cyclic AMP-regulated exocytosis of Escherichia coli from infected bladder epithelial cells. Nat Med 2007;13:625-30. PMID: 17417648



24. Jorgensen I, Seed PC. How to make it in the urinary tract: a tutorial by Escherichia coli. PLoS Pathog 2012;8:e1002907. PMID: 23055921



25. Hunstad DA, Justice SS. Intracellular lifestyles and immune evasion strategies of uropathogenic Escherichia coli. Annu Rev Microbiol 2010;64:203-21. PMID: 20825346


26. Ulett GC, Valle J, Beloin C, Sherlock O, Ghigo JM, Schembri MA. Functional analysis of antigen 43 in uropathogenic Escherichia coli reveals a role in long-term persistence in the urinary tract. Infect Immun 2007;75:3233-44. PMID: 17420234




27. Hannan TJ, Totsika M, Mansfield KJ, Moore KH, Schembri MA, Hultgren SJ. Host-pathogen checkpoints and population bottlenecks in persistent and intracellular uropathogenic Escherichia coli bladder infection. FEMS Microbiol Rev 2012;36:616-48. PMID: 22404313



28. Elliott TS, Reed L, Slack RC, Bishop MC. Bacteriology and ultrastructure of the bladder in patients with urinary tract infections. J Infect 1985;11:191-9. PMID: 3936879


29. Sharma K, Dhar N, Thacker VV, Simonet TM, Signorino-Gelo F, Knott GW, et al. Dynamic persistence of UPEC intracellular bacterial communities in a human bladder-chip model of urinary tract infection. Elife 2021;10:e66481. 

30. Valentine-King MA, Trautner BW, Zoorob RJ, Salemi JL, Gupta K, Grigoryan L. Predicting antibiotic susceptibility among patients with recurrent urinary tract infection using a prior culture. J Urol 2024;211:144-52. PMID: 37820311


31. Jeng SL, Huang ZJ, Yang DC, Teng CH, Wang MC. Machine learning to predict the development of recurrent urinary tract infection related to single uropathogen, Escherichia coli. Sci Rep 2022;12:17216. PMID: 36241875




32. Hannan TJ, Roberts PL, Riehl TE, van der Post S, Binkley JM, Schwartz DJ, et al. Inhibition of cyclooxygenase-2 prevents chronic and recurrent cystitis. EBioMedicine 2014;1:46-57. PMID: 26125048



33. Nseir W, Taha M, Nemarny H, Mograbi J. The association between serum levels of vitamin D and recurrent urinary tract infections in premenopausal women. Int J Infect Dis 2013;17:e1121-4. PMID: 23911156


34. Singer E, Markó L, Paragas N, Barasch J, Dragun D, Müller DN, et al. Neutrophil gelatinase‐associated lipocalin: pathophysiology and clinical applications. Acta Physiol (Oxf) 2013;207:663-72. PMID: 23375078



35. Forster CS, Johnson K, Patel V, Wax R, Rodig N, Barasch J, et al. Urinary NGAL deficiency in recurrent urinary tract infections. Pediatr Nephrol 2017;32:1077-80. PMID: 28210838




36. Jhang JF, Kuo HC. Recent advances in recurrent urinary tract infection from pathogenesis and biomarkers to prevention. Ci Ji Yi Xue Za Zhi 2017;29:131-7. PMID: 28974905



37. Soto SM. Importance of biofilms in urinary tract infections: new therapeutic approaches. Adv Biol 2014;2014:543974. 

38. Manfiolli AO, Dos Reis TF, de Assis LJ, de Castro PA, Silva LP, Hori JI, et al. Mitogen activated protein kinases (MAPK) and protein phosphatases are involved in Aspergillus fumigatus adhesion and biofilm formation. Cell Surf 2018;1:43-56. PMID: 32743127



39. Liang X, Chen YY, Ruiz T, Wu H. New cell surface protein involved in biofilm formation by Streptococcus parasanguinis. Infect Immun 2011;79:3239-48. PMID: 21576336




40. Hartford O, O’Brien L, Schofield K, Wells J, Foster TJ. The Fbe (SdrG) protein of Staphylococcus epidermidis HB promotes bacterial adherence to fibrinogen. Microbiology (Reading) 2001;147(Pt 9):2545-52. PMID: 11535794


41. Pani A, Valeria L, Dugnani S, Senatore M, Scaglione F. Pharmacodynamics of D-mannose in the prevention of recurrent urinary infections. J Chemother 2022;34:459-64. PMID: 35416116


42. Mulvey MA, Schilling JD, Hultgren SJ. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect Immun 2001;69:4572-9. PMID: 11402001