 |
 |


- Search
| Int Neurourol J > Volume 24(2); 2020 > Article |
|
ABSTRACT
Purpose
Alpha1-adrenoceptors participate in improving storage symptoms of male lower urinary tract symptoms (LUTS). However, the mechanism of action of these compounds remains unclear. To clarify the mechanism of the α1-adrenoceptor antagonists, the amplitude of miniature excitatory postsynaptic currents (mEPSCs) was analyzed in the lumbosacral spinal cord in rats.
Methods
Male adult Sprague-Dawley rats were used. Blind whole-cell patch-clamp recordings were performed on substantia gelatinosa (SG) neurons in spinal cord slice preparations. The amplitude of mEPSCs was recorded in individual SG neurons to which α1-adrenoceptors (100μM naftopidil, 100μM tamsulosin, and 30μM silodosin) were applied sequentially with intervening washout periods. Individual amplitudes were analyzed.
Results
Pearson correlation coefficients (r) for the amplitudes of mEPSCs between the baseline and postadministration of α1-adrenoceptor antagonists indicated changes of the amplitude ranked in the order of naftopidil (r =0.393), tamsulosin (r=0.738), and silodosin (r=0.944). Together, the α1-adrenoceptor antagonists yielded significant increases in the amplitude of mEPSCs in SG neurons (n=108, P=0.012). However, the effects of each α1-adrenoceptor antagonist on the amplitude were as follows (relative to the baseline; n=36 each): naftopidil, P=0.129; tamsulosin, P=0.201; and silodosin, P=0.005. The rate of response to naftopidil for the outward current was relatively high among the α1-adrenoceptor blockers. An inward current was observed only with the naftopidil application.
Conclusions
Alpha1-adrenoceptor antagonists changed the amplitudes of mEPSCs in a subset of SG neurons in slices prepared from the L6–S1 levels of rat spine. Although the α1-adrenoceptor antagonists generated inward or outward currents in the SG neurons, different rates of response were observed with each antagonist. These results are important for understanding the mechanisms of action (at the spinal level) of α1-adrenoceptor antagonists for the storage symptoms of male LUTS.
- Alpha1-adrenoceptor antagonists changed the amplitude of miniature excitatory postsynaptic currents in a subset of substantia gelatinosa neurons by slice patch-clamp technique in rat spinal cord.
- The magnitude of the changes ranked (in descending order) from naftopidil to tamsulosin to silodosin.
Lower urinary tract symptoms (LUTS) consist of voiding, storage, and postmicturition symptoms [1]. Male LUTS is related to a variety of causes, including, for example, benign prostatic obstruction/benign prostatic hyperplasia, bladder dysfunction including overactive bladder, and nocturnal polyuria [2]. The prevalence of LUTS increases with age, and a considerable proportion of men are affected by LUTS [3]. To manage male LUTS pharmacologically, α1-adrenoceptor antagonists, 5α-reductase inhibitors, phosphodiesterase 5 inhibitors, and plant extracts are prescribed. Among these treatments, α1-adrenoceptor blockers are the most established drugs and are widely prescribed and taken. α1-adrenoceptor antagonists act by relaxing smooth muscle in the urethra and prostate, resulting in a reduction of enhanced tonus or contractility, thus counteracting decreased urine flow rates [4]. Additionally, α1-adrenoceptors contribute to the improvement of storage symptoms [5], although the mechanism of action of these effects remains unclear. In rats subjected to conscious cystometry, tamsulosin, naftopidil, and silodosin were shown to prolong the micturition interval [6]. When isovolumetric cystometry was conducted in anesthetized animals, intrathecally administered naftopidil yielded increased inter-contraction intervals [7]. Interestingly, the effect of naftopidil was counteracted by bicuculline and/or glycine injected intrathecally [8]. Clinical studies have yielded contradictory results in comparisons between naftopidil and tamsulosin with evaluation by the International Prostate Symptom Score [9] and by urodynamic parameters [10]. The differences between the 2 studies were thought to reflect some aspect of the mode of action (e.g., a selectivity for subtypes of α1-adrenoceptors), but the actual basis of these differences remains unknown.
By using voltage-clamp recordings, it is possible to identify primary afferent information in substantia gelatinosa (SG, lamina II of Rexed) neurons [11], effects that are mediated by different primary afferent fibers [12,13]. The efficacy of synaptic transmission is determined by presynaptic neurotransmitter release probability and postsynaptic responsiveness, parameters that are evaluated by the frequency and amplitude of miniature excitatory or inhibitory postsynaptic currents (mEPSC or mIPSC), respectively. A slice patch-clamp recording can measure miniature excitatory and inhibitory postsynaptic currents, which are thought to reflect the following details. The frequency of the current indicates presynaptic events, which correspond to changes in the probability of neurotransmitter release and changes in the number of synapses [14]. Meanwhile, the amplitude of the current indicates postsynaptic information, corresponding to changes in the number of receptors for neurotransmitters on the postsynaptic site. When outward/inward currents are observed, the recording neuron shows depolarization or hyperpolarization by activation of receptors for neurotransmitters at postsynaptic sites, yielding changes in membrane potential [15]. Analyses of frequency and amplitude distributions of mEPSCs permit determination of the loci of experimental manipulation (i.e., presynaptic and/or postsynaptic) [16]. The effects of naftopidil, tamsulosin, and silodosin on the frequency of excitatory synaptic currents at synaptic terminal sites in the spinal cord have been determined [17], and an explanation for the diversity of efficacy in treatment of storage symptoms by α1-adrenoceptor antagonists is being developed. Naftopidil inhibits the amplitudes of EPSCs evoked by dorsal root stimulation and increases the frequencies of mEPSCs [18]. However, the effect of naftopidil on the amplitudes of mEPSCs, meaning upregulation of sensitivity for depolarization of postsynaptic cells, has not yet been described (to our knowledge). Therefore, the goal of the present study was to clarify the effect of naftopidil on the amplitudes of the mEPSCs in SG neurons derived from lumbosacral spinal cord in rats and to explain the mechanism of action of α1-adrenoceptor antagonists in the treatment of storage symptoms.
The methods for obtaining slices of the adult rat spinal cord and for blind patch-clamp recordings from SG neurons have been described in detail elsewhere [17,19]. Briefly, adult male SpragueDawley rats (6–8 weeks old) were deeply anesthetized with urethane (1.2 g/kg, intraperitoneally), and a lumbosacral laminectomy then was performed. The lumbosacral segments of the spinal cord (L2–S3), with the associated ventral and dorsal roots, were removed and placed in ice-cold Krebs solution equilibrated with 95% O2–5% CO2. The Krebs solution contained (in mM): NaCl 117, KCl 3.6, CaCl2 2.5, MgCl2 1.2, NaH2PO4 1.2, NaHCO3 25, and glucose 11 (pH, 7.4). Immediately after removal of the spinal cord, the rats were killed by exsanguination under urethane anesthesia. The pia-arachnoid membrane was removed after cutting all of the ventral and dorsal roots. The spinal cord was mounted on a vibratome, and a 500-µm-thick transverse slice with the attached dorsal root was cut. The slice was placed on a nylon mesh in the recording chamber in a volume of 0.5-mL Krebs solution, and the slice was completely submerged in and perfused with Krebs solution saturated with 95% O2–5% CO2 at 37°C±1°C and a flow rate of 10–15 mL/min.
The SG was easily discernible with transmitted illumination as a relatively translucent band across the dorsal horn in the transverse slice preparations (Fig. 1). Blind whole-cell voltage-clamp recordings were made from SG neurons, as previously described [17,19]. The patch pipettes were filled with a solution containing potassium gluconate solution (in mM): K-gluconate 135, KCl 5, CaCl2 0.5, MgCl2 2, EGTA 5, HEPES 5, and ATPMg 5 (pH 7.2). The tip resistance of the patch pipettes was 6–12 MΩ. Series resistance was assessed according to the response to a 5-mV hyperpolarizing step. This value was monitored during the recording session, and data were rejected if values changed by >15%. Signals were acquired with a patch-clamp amplifier (Axopatch 700A, Molecular Devices, Union City, CA, USA). The data were digitized with an AD/DA converter (Digidata 1321A, Molecular Devices), stored on a personal computer using a data acquisition program (Clampex, version 9.0, Molecular Devices), and analyzed using a software package (Clampfit, version 9.0, Molecular Devices) (Fig. 1). Cell recordings were obtained in voltage-clamp mode at holding potentials of -70 mV to record EPSCs [17,19].
1-[4-(2-methoxyphenyl) piperazinyl]-3-(1-naphthyloxy) propan-2-ol (naftopidil) (PubChem CID: 4418) (Asahi Kasei Pharma Co., Tokyo, Japan) was dissolved in 1% dimethyl sulfoxide (DMSO) (PubChem CID: 679) (Wako, Osaka, Japan) in Krebs solution. Tamsulosin and silodosin were dissolved in Krebs solution. All drugs were applied by sequential perfusion in a single cell with washout periods via a three-way stopcock without changes in the perfusion rate or temperature. The application schedule was as described previously [17].
Statistical analysis was performed using JMP ver. 14 (SAS Institute, Cary, NC, USA), and P-values <0.05 were considered statistically significant. The analyses consisted of 4 sets of tests as described below.
For individual SG neurons, the amplitude after administration of naftopidil, tamsulosin, or silodosin was plotted against amplitude before administration of the respective reagent. For each α1-adrenoceptor antagonist, correlations were subjected to linear regression, and the values of r2 and the slopes of the regression lines were calculated. Pearson correlation coefficients (r) were analyzed for point-estimates and 95% confidence intervals (CIs) using 2-tailed tests.
For global analysis, amplitudes after administration of all α1-adrenoceptor antagonists were compared to those before administration of the blockers by using a 2-tailed paired t-test. If the statistical significance was confirmed in the global analysis, the differences in changes of amplitude were compared between each α1-adrenoceptor antagonist in the same SG neuron, again using a 2-tailed paired t-test. Additionally, differences in the amplitudes before and after administration were compared for naftopidil, tamsulosin, and silodosin using the 2-tailed Tukey-Kramer test.
To compare the extent of changes in amplitudes by naftopidil, tamsulosin, and silodosin in individual SG neurons, waterfall plots were generated for the differences in amplitudes between the before-and after-administration values for each α1-adrenoceptor antagonist. The effects of each antagonist were plotted as histograms of values in descending order of difference.
Typical mEPSC recordings of changes of amplitude in response to α1-adrenoceptor antagonist exposure are provided in Fig. 2, including examples that exhibited changes in frequency and amplitude either without inducing outward and/or inward current (A), with inward current induced (B), or with outward current induced (C).
Pearson correlation coefficients for the amplitudes between the baseline and postadministration of α1-adrenoceptor antagonists ranked (in decreasing order) as naftopidil (r=0.393; 95% CI, 0.074–0.639), tamsulosin (r=0.738; 95% CI, 0.541–0.859), and silodosin (r=0.944; 95% CI, 0.891–0.971). For the correlation coefficient, the extent of lowering from 1.000 corresponds to potency of the drug. For naftopidil, tamsulosin, and silodosin, the regression lines yielded values for slopes of 0.573, 1.046, and 1.143, respectively (Fig. 3).
Global analysis showed that the α1-adrenoceptor antagonists yielded significant increases in the amplitude of mEPSCs of SG neurons, which rose from 11.8 pA at baseline to 13.0 pA following exposure (n=108, P=0.012) (Table 1). However, only silodosin demonstrated a significant elevation (relative to the baseline) when the data were broken down by antagonist (n= 36 each) from 11.7 pA to 12.5 pA (P=0.005). Naftopidil and tamsulosin tended to increase the amplitudes but without statistical significance with individual effects as follows: naftopidil, from 12.1 pA to 13.9 pA (P=0.129); tamsulosin, from 11.7 pA to 12.5 pA (P=0.201) (Table 1). Additionally, statistical significances were not found among α1-adrenoceptor antagonists for the changes of amplitude before and after application of the antagonists to a given SG neuron (Table 1).
As shown in Fig. 4, the waterfalls plots of naftopidil, tamsulosin, and silodosin permitted apparent ranking by the strength of the effects. The changes of amplitudes of mEPSCs for naftopidil ranged from positive to negative, while those for silodosin exhibited a narrower range.
Observability of inward or outward current following α1-adrenoceptor antagonist exposure differed among naftopidil (total: 9 out of 36 cells, 25.0%; inward: 3 cells, 8.3%; outward: 6 cells, 16.7%), tamsulosin (total: 5 out of 36 cells, 13.9%; outward only), and silodosin (total: 2 out of 36 cells, 5.6%; outward only). The values of the currents are summarized in Table 2.
In individual SG neurons, each of 3 α1-adrenoceptor antagonists was evaluated sequentially with mEPSC recording by the patch-clamp technique. The amplitudes of the mEPSCs were analyzed and the descriptive statistics values were noted. Various types of responsiveness to each α1-adrenoceptor antagonists were observed (Fig. 4). The responsiveness to naftopidil was twice that to tamsulosin (25% vs. 13.9%), and 5 times that to silodosin (25% vs. 5.6%), as shown in Table 2.
For tamsulosin and silodosin, Pearson correlation coefficients for the amplitudes between baseline and after the application were 0.738 and 0.944 (respectively), implying strong correlation. In contrast, the value for naftopidil was 0.393, suggesting weak correlation. These results indicated that the changes of amplitude of mEPSCs between before and after the application of α1-adrenoceptor antagonists are small for tamsulosin or silodosin and large for naftopidil. Furthermore, since a change in the amplitude of mEPSCs reveals a change in sensitivity for postsynaptic excitation (e.g., an upregulation of receptors for glutamate), naftopidil may promote changes in sensitivity, an effect not seen with tamsulosin and silodosin.
Our global analysis indicated that α1-adrenoceptor antagonists increase the amplitude of the mEPSCs of SG neurons. We hypothesize that the effects of naftopidil and tamsulosin were not statistically significant because the numbers of SG neurons that responded to naftopidil or tamsulosin were low. For instance, if the responsive SG neurons are defined as those in which the amplitude (pA) of mEPSCs change by>20% or<-20% (relative to the baseline), then 8 would be considered responsive to naftopidil and 5 each would be considered responsive to tamsulosin or silodosin (out of 36 SG neurons each; data not shown). In terms of the differences (∆) in amplitude, the effect size was smallest in the silodosin group (Table 1). Therefore, we infer that the smaller variance in the amplitude of mEPSCs was the reason that statistical significance was observed after application of silodosin. The ranges of responses observed in the amplitudes differed among the α1-adrenoceptor antagonists, as shown in the waterfall plots (Fig. 3). The waterfall plots for tamsulosin and silodosin resembled each other, which may reflect the small difference in the Pearson correlation coefficients for these 2 antagonists (0.738 and 0.944, respectively). Although the increases in the amplitude of mEPSCs with naftopidil and tamsulosin fell short of statistical significance, the responsive SG neurons (i.e., those whose amplitude (in pA) increased or decreased by more than 20% relative to the baseline) represented a subpopulation (among 36 SG neurons each) of 8 for naftopidil and 5 for tamsulosin neurons (data not shown). These results suggested that the neurons responsive to specific α1-adrenoreceptor blockers are present in defined proportions. Given that mEPSCs represent glutamatergic transmission in afferent fibers [20], increases in the amplitude of mEPSCs suggest upregulation of the sensitivity of receptors that mediate glutamatergic transmission, which in turn would be expected to facilitate inhibitory GABAergic and/or glycinergic interneurons. Although silodosin exposure yielded a significant increase in the amplitude of mEPSCs (Table 1), the magnitude of the effect was small. Therefore, silodosin may have a limited effect on SG neurons.
Previous work showed that naftopidil suppresses the amplitude of mEPSCs evoked by dorsal root stimulation in the SG neurons of slices isolated from the lumbosacral spine, while prazosin does not [21]. Those results indicated that naftopidil may attenuate the excitation caused by primary afferent fibers at the postsynaptic site without blocking α1-adrenoceptors. Furthermore, naftopidil facilitated larger changes in the frequencies of mEPSCs in SG neurons of slices isolated from the same level of spine than did tamsulosin or silodosin [17]. This distinction suggests that naftopidil’s ability to suppress the micturition reflex may result from excitation of postsynaptic cells via an elevated frequency of neurotransmitter release from the presynaptic sites, facilitating inhibition in subsequent inhibitory interneurons. The present study indicated that naftopidil increased and decreased the amplitude of the mEPSCs in SG neurons (Fig. 4). The increases suggest upregulation of the sensitivity at the postsynaptic site for the neurotransmitter (e.g., GABA, glycine) released from the presynaptic terminal. The decreases suggest downregulation of the sensitivity at the postsynaptic site for the neurotransmitter (e.g., glutamate) released from the presynaptic terminal. These bidirectional effects suggest that increases and decreases in amplitude may reflect the excitation and sedation (respectively) of postsynaptic cells.
In the present study, the α1-adrenoceptor antagonists generated inward or outward currents in a subset of SG neurons; notably, the outward current was observed more readily than was the inward current (Table 2). We speculate that the 3 antagonists directly facilitate hyperpolarization of SG neurons by generating outward currents. In view of the inward current seen for mEPSCs, it is possible that naftopidil encourages depolarization in a small subpopulation of the SG neurons. These results suggest that naftopidil modulates neurotransmission in the SG neurons multimodally.
It remains difficult, using the current technique, to determine an actual relationship between the amplitude of EPSC and the storage symptoms. At minimum, in vivo electrophysiological recording would need to be combined with cystometry, which would have to be performed under anesthesia. As far as we know, correlations between urinary bladder activity and afferent nerve activity, but not between bladder activity and EPSC, have been reported, in which action potentials of afferent fibers isolated from the left L6 dorsal root were recorded [22]. In the previous study on EPSC, a correlation between the amplitude and the afferent nociceptive input was reported [19]. There is a substantial difference between painful stimuli and storage symptoms, but both are aspects of the afferent sensory nerve. Therefore, we speculate that the amplitude of EPSC may modulate the storage symptoms.
During the storage of urine, the storage reflex generated by primary afferent firing is coordinated by the spinal reflex pathway [23]. In human functional analysis, bladder activity is mediated by stimulation of brain subregions (for example, the periaqueductal gray matter [24]). In the lumbosacral region, the SG (lamina II) of the spinal dorsal horn contains a high density of excitatory and inhibitory interneurons that are thought to be critically involved in the modulation of nociception [25] and (presumably) the micturition reflex. Sensory information is carried from the pelvic organs to the dorsal horn of the lumbosacral spinal cord [26]. In the rat spinal cord, glutamatergic mechanisms play an essential role in micturition control [27]. Intrathecal injection of α1-adrenoceptor antagonists has been shown to inhibit the micturition reflex in animal models [7,28]. These results suggest that the α1-adrenoceptor antagonists exert their activity at the spinal level in the neural circuitry. In the present study, several neurons exhibited increased or decreased amplitudes of mEPSCs in response to α1-adrenoceptor antagonists, particularly naftopidil (Fig. 4). These results indicated that the postsynaptic sites of various SG neurons have differing sensitivities for excitatory neurotransmitters (e.g., glutamate). Although the physiological significance of the amplitude of the EPSC has not been determined to date, the 3 α1-adrenoceptor antagonists tested in the present study are presumed to exert their activities in the postsynaptic site, while prazosin apparently does not [21]. As shown in the waterfall plots of the present study, neurons that responded to the α1-adrenoceptor antagonists predominantly exhibited increased (and not decreased) amplitudes of mEPSCs. Therefore, we speculate that naftopidil and tamsulosin suppress micturition by upregulating sensitivity at the postsynaptic site of inhibitory interneurons, thereby activating inhibitory neurotransmission. Additionally, silodosin may contribute in part by activating the inhibitory pathway in the spinal cord.
In conclusion, although exposure to α1-adrenoceptor antagonists increased the amplitudes of mEPSCs in rat SG neurons in slices prepared from the L6–S1 spine level, these effects reflected responses in a subset of the SG neurons. Additionally, the α1-adrenoceptor antagonists generated inward/outward currents in the SG neurons. The magnitude of the changes induced by the α1-adrenoceptor antagonists ranged (in descending order) from naftopidil to tamsulosin to silodosin. These results are expected to increase our understanding of the mechanisms of action (at the spine level) of α1-adrenoceptor antagonists when used clinically for the treatment of storage symptoms associated with LUTS.
This study is phenomenologically based; therefore, further electrophysiological and molecular biological investigations of the proposed mechanisms will be needed. Although afferent nerves from the urothelium largely project to lamina X in the dorsal horn, SG neurons in lamina II were used. As described previously, a part of the superficial neurons receives nociceptive and non-nociceptive inputs from the lower urinary tract, as shown by upregulation of cFos expression [29]. Although the use of lamina X would be ideal, that structure possesses a low density of neurons, making the blind attachment of patchclamp electrodes less efficient. Therefore, lamina II was examined. Furthermore, our results indicated that α1-adrenoceptor antagonists alter the amplitudes of mEPSCs in only a subset of SG neurons. This observation is consistent with the existence of multiple types of SG neurons within this lumbosacral level [30].
NOTES
Grant/Fund Support
This work was supported by grants from the programs for Grants-in-Aid for Scientific Research (DU and MY) of the Ministry of Education, Science, Sports and Culture of Japan (Grant Numbers JP19K09323, JP15K08667, JP25860431, and JP21600005), and was partially supported by the MEXT-Supported Program for the Strategic Research Foundation at Private Universities, “Creation of 3D nano-micro structures and its application to biomimetics and medicine”, during 2015-2019 (DU, Grant Number S1511031). This study also was supported in part by the Asahi Kasei Pharma Corporation.
Research Ethics
All experiments were performed in accordance with the “Guiding Principles for Care and Use of Animals in the Field of Physiological Sciences” of the Physiological Society of Japan and were approved by the local Animal Experiment Committee of the Kumamoto Health Science University and Kyushu University. All efforts were made to minimize animal suffering and the number of animals used for the studies.
REFERENCES
1. Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology 2003;61:37-49. PMID: 12559262


2. Management of non-neurogenic male LUTS [Internet]; Arnhem (The Netherlands): European Association of Urology; 2018 [cited 2018 Dec 8]. Available from: https://uroweb.org/guideline/treatment-ofnon-neurogenic-male-luts/.
3. Rohrmann S, Katzke V, Kaaks R. Prevalence and progression of lower urinary tract symptoms in an aging population. Urology 2016;95:158-63. PMID: 27346671


4. Takei R, Ikegaki I, Shibata K, Tsujimoto G, Asano T. Naftopidil, a novel alpha1-adrenoceptor antagonist, displays selective inhibition of canine prostatic pressure and high affinity binding to cloned human alpha1-adrenoceptors. Jpn J Pharmacol 1999;79:447-54. PMID: 10361884


5. Kwon SY, Lee KS, Yoo TK, Chung JI, Lee JY, Hong JH, et al. Comparison of the effect of naftopidil 75 mg and tamsulosin 0.2 mg on the bladder storage symptom with benign prostatic hyperplasia: prospective, multi-institutional study. Urology 2018;111:145-50. PMID: 28624553


6. Chen Z, Ishizuka O, Imamura T, Aizawa N, Igawa Y, Nishizawa O, et al. Role of alpha1-adrenergic receptors in detrusor overactivity induced by cold stress in conscious rats. Neurourol Urodyn 2009;28:251-6. PMID: 18837433


7. Sugaya K, Nishijima S, Miyazato M, Ashitomi K, Hatano T, Ogawa Y. Effects of intrathecal injection of tamsulosin and naftopidil, alpha-1A and -1D adrenergic receptor antagonists, on bladder activity in rats. Neurosci Lett 2002;328:74-6. PMID: 12123863


8. Sugaya K, Nishijima S, Kadekawa K, Ashitomi K, Ueda T, Yamamoto H. Spinal mechanism of micturition reflex inhibition by naftopidil in rats. Life Sci 2014;116:106-11. PMID: 25258114


9. Gotoh M, Kamihira O, Kinukawa T, Ono Y, Ohshima S, Origasa H, et al. Comparison of tamsulosin and naftopidil for efficacy and safety in the treatment of benign prostatic hyperplasia: a randomized controlled trial. BJU Int 2005;96:581-6. PMID: 16104914


10. Nishino Y, Masue T, Miwa K, Takahashi Y, Ishihara S, Deguchi T. Comparison of two alpha1-adrenoceptor antagonists, naftopidil and tamsulosin hydrochloride, in the treatment of lower urinary tract symptoms with benign prostatic hyperplasia: a randomized crossover study. BJU Int 2006;97:747-51 discussion 751. PMID: 16536766


11. Rexed B. The cytoarchitectonic organization of the spinal cord in the cat. J Comp Neurol 1952;96:414-95. PMID: 14946260


12. Yang K, Feng Y, Li Y. Baclofen inhibition of dorsal root-evoked inhibitory postsynaptic currents in substantia gelatinosa neurons of rat spinal cord slice. Brain Res 2001;900:320-3. PMID: 11334813


13. Yang K, Kumamoto E, Furue H, Li YQ, Yoshimura M. Action of capsaicin on dorsal root-evoked synaptic transmission to substantia gelatinosa neurons in adult rat spinal cord slices. Brain Res 1999;830:268-73. PMID: 10366683


14. Ikeda K, Satake S, Onaka T, Sugimoto H, Takeda N, Imoto K, et al. Enhanced inhibitory neurotransmission in the cerebellar cortex of Atp1a3-deficient heterozygous mice. J Physiol 2013;591:3433-49. PMID: 23652595



15. Xu ZH, Wang C, Fujita T, Jiang CY, Kumamoto E. Action of thymol on spontaneous excitatory transmission in adult rat spinal substantia gelatinosa neurons. Neurosci Lett 2015;606:94-9. PMID: 26314510


16. Yang K, Li YQ. Origins of spontaneous and noxious stimuli-evoked miniature EPSCs in substantia gelatinosa. Neuroreport 2001;12:39-42. PMID: 11201088


17. Uta D, Hattori T, Yoshimura M. Characterization on responsiveness of excitatory synaptic transmissions to α1-adrenoceptor blockers in substantia gelatinosa neurons isolated from lumbosacral level in rat spinal cords. Int Neurourol J 2019;23:13-21. PMID: 30943690




18. Uta D, Xie DJ, Hattori T, Kasahara KI, Yoshimura M. Effects of naftopidil on inhibitory transmission in substantia gelatinosa neurons of the rat spinal dorsal horn in vitro. J Neurol Sci 2017;380:205-11. PMID: 28870570


19. Uta D, Kato G, Doi A, Andoh T, Kume T, Yoshimura M, et al. Animal models of chronic pain increase spontaneous glutamatergic transmission in adult rat spinal dorsal horn in vitro and in vivo. Biochem Biophys Res Commun 2019;512:352-9. PMID: 30894274


20. Guo YX, Li DP, Chen SR, Pan HL. Distinct intrinsic and synaptic properties of pre-sympathetic and pre-parasympathetic output neurons in Barrington’s nucleus. J Neurochem 2013;126:338-48. PMID: 23647148



21. Uta D, Hattori T, Yoshimura M. Effects of high concentrations of naftopidil on dorsal root-evoked excitatory synaptic transmissions in substantia gelatinosa neurons in vitro. Int Neurourol J 2018;22:252-9. PMID: 30599496




22. Aizawa N, Sugiyama R, Ichihara K, Fujimura T, Fukuhara H, Homma Y, et al. Functional roles of bladder α1-adrenoceptors in the activation of single-unit primary bladder afferent activity in rats. BJU Int 2016;117:993-1001. PMID: 26332379


23. de Groat WC. Integrative control of the lower urinary tract: preclinical perspective. Br J Pharmacol 2006;147 Suppl 2(Suppl 2):S25-40. PMID: 16465182


24. Griffiths D, Derbyshire S, Stenger A, Resnick N. Brain control of normal and overactive bladder. J Urol 2005;174:1862-7. PMID: 16217325


25. Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci 2010;11:823-36. PMID: 21068766




27. Yoshiyama M, de Groat WC. Supraspinal and spinal alpha-amino3-hydroxy-5-methylisoxazole-4-propionic acid and N-methyl-D-aspartate glutamatergic control of the micturition reflex in the urethane-anesthetized rat. Neuroscience 2005;132:1017-26. PMID: 15857706



28. Yoshizumi M, Matsumoto-Miyai K, Yonezawa A, Kawatani M. Role of supraspinal and spinal alpha1-adrenergic receptor subtypes in micturition reflex in conscious rats. Am J Physiol Renal Physiol 2010;299:F785-91. PMID: 20668103


29. Birder LA, de Groat WC. Increased c-fos expression in spinal neurons after irritation of the lower urinary tract in the rat. J Neurosci 1992;12:4878-89. PMID: 1464772



Fig. 1.
The experimental method and design are illustrated. Blind whole-cell patch-clamp recording was performed in individual substantia gelatinosa neurons of an adult rat spinal dorsal horn. A/D, analog to digital.
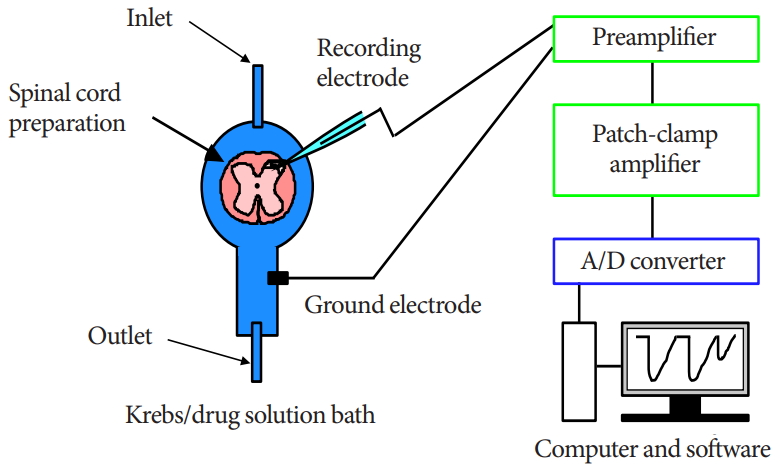
Fig. 2.
Charts of typical mEPSCs are presented. (A) Frequency and amplitude were changed but outward/inward currents were not altered. (B, C) Inward current and outward currents (respectively) were observed. The bar indicates the time of application of the α1-adrenoceptor blocker.

Fig. 3.
Correlations of the amplitudes of mEPSCs between baseline and postadministration of α1-adrenoceptor antagonists. n=36. mEPSC, miniature excitatory postsynaptic current.
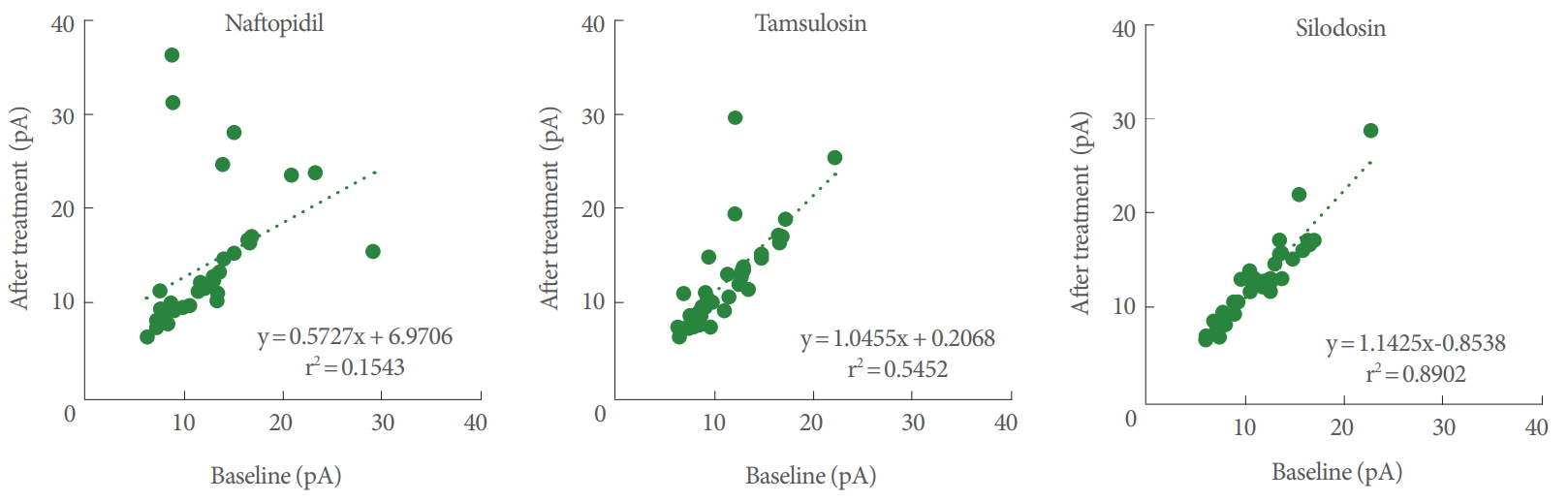
Fig. 4.
Waterfall plots for the difference of amplitudes of mEPSCs between baseline and postadministration of α1-adrenoceptor antagonists. The values of the differences were aligned in descending order. n=36. mEPSC, miniature excitatory postsynaptic current.
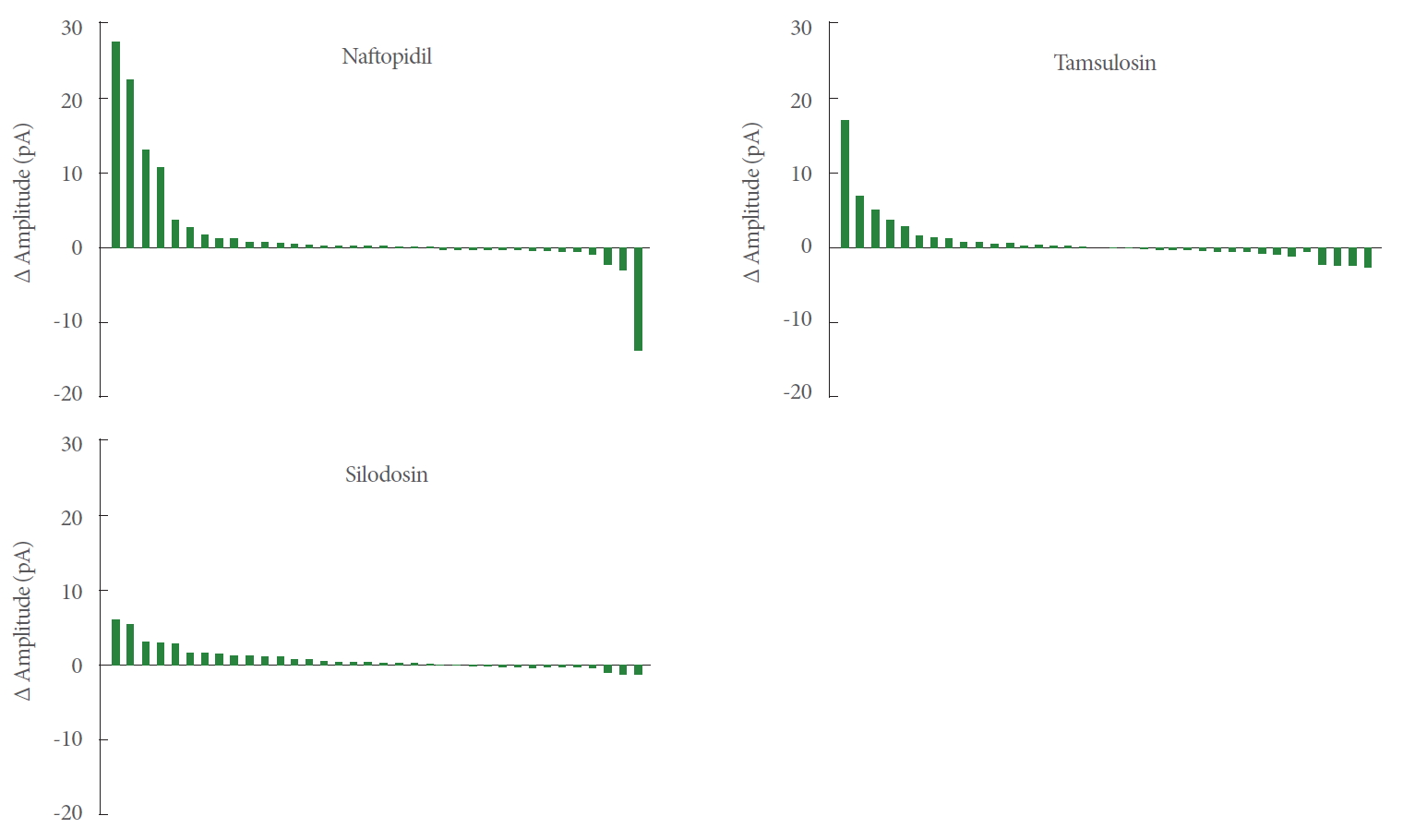
Table 1.
The effects of α1-adrenoceptor antagonists on the amplitude of EPSC in SG neurons of slices prepared from the lumbosacral level in rats
Values are presented as means±standard error of the mean with 95% confidence interval in parentheses.
Statistical analyses were performed by 2-tailed paired Student t-tests to compare the values between baseline and after treatment in a given neuron, and by 2-tailed Turkey-Kramer test to compare the values among the groups treated with distinct α1-adrenoceptor antagonists.
EPSC, excitatory post synaptic current; SG, substantia gelatinosa.
Table 2.
The values of inward/outward currents generated by each α1-adrenoceptor antagonist





