Stem Cell Therapy for Neurogenic Bladder Dysfunction in Rodent Models: A Systematic Review
Article information
Abstract
Purpose
Neurogenic bladder dysfunction (NGB) has an impact on the quality of life, which made it an important research subject in preclinical studies. The present review investigates the effect of stem cell (SC) therapy on bladder functional recovery after the onset of spinal cord injury (SCI), multiple sclerosis (MS), Parkinson disease (PD), and stroke in rodent models.
Methods
All experiments evaluated the regenerative potential of SC on the management of NGB in rodent models up to June 2019, were included. From 1,189 relevant publications, 20 studies met our inclusion criteria of which 15 were conducted on SCI, 2 on PD, 2 on stroke, and 1 on MS in the rodent models. We conducted a meta-analysis on SCI experiments and for other neurological diseases, detailed urodynamic findings were reported.
Results
The common SC sources used for therapeutical purposes were neural progenitor cells, bone marrow mesenchymal SCs, human amniotic fluid SCs, and human umbilical cord blood SCs. There was a significant improvement of micturition pressure in both contusion and transaction SCI models 4 and 8 weeks post-SC transplantation. Residual urine volume, micturition volume, and bladder capacity were improved 28 days after SC transplantation only in the transaction model of SCI. Nonvoiding contraction recovered only in 56 days post-cell transplantation in the contusion model.
Conclusions
Partial bladder recovery has been evident after SC therapy in SCI models. Due to limitations in the number of studies in other neurological diseases, additional studies are necessary to confirm the detailed mechanism for bladder recovery.
INTRODUCTION
Neurogenic bladder (NGB) is an ineffective bladder that originated from the damage to the central or peripheral nervous system. Regarding the site and severity of injury to the nervous system, patients usually experience urinary frequency, incontinence, urgency, and urinary tract infection [1]. In the United States, a fraction of patients with multiple sclerosis (MS; 40%– 90%), Parkinson disease (PD; 37%–72%), and stroke (15%) suffer from NGB. In the 50%–90% of patients with MS, hyperreflexia is indicated and the others have areflexia [2]. After spinal cord injury (SCI), 70%–84% of patients confront with some degree of bladder dysfunction [3]. NGB has a tremendous effect on the quality of life [4]. In addition to physical and clinical aspects, the signs associated with urinary incontinence can negatively impact a patient’s quality of life [5]. Different strategies including conservative methods (lifestyle changes, bladder retraining, pelvic floor muscle training), pharmacological (anticholinergics, β-adrenoceptor agonists) and nonpharmacological approaches (electrical stimulation, clean intermittent catheterization, and indwelling catheters), as well as surgical interventions (augmentation cystoplasty), have been developed for the management of NGB. However, improvement in voiding dysfunction is not been fully achieved and also was accompanied by several side effects [4,6-10]. Based on the results from previous experiments, stem cell (SC) transplantation is used in the management of neuro-urological diseases accompanied by promising outcomes [11]. The potential of self-renewal, multilineage differentiation, site-specific migration, tissue regeneration, made SC as a beneficial therapeutic target in the treatment of several types of complications such as degenerative diseases [12]. The major issues in SC therapy correlate with cell survival, dynamic growth, and regeneration potential of transplanted cells in the long-term outcome [13]. Despite the promising effects of SC therapies, there is a concern about cellular rejection by the adaptive immune response in the host tissues. A large number of transplant cells die in the early hours to days postadministration [14]. Animal models are valuable tools for evaluating new therapeutic agents and efficacy of candidate interventions such as SC. Also, rodent models mimic many features of disorders and have been extensively applied to study the mechanisms of therapeutic interventions and assess the possible adverse effects of treatments [15]. Considering the possible unwanted effects associated with SC administration, appropriate integration to the host tissues, and a number of viable SCs, it limits the efficacy and therapeutic potential of this approach [16]. Despite these challenges, SC therapy makes significant steps toward the clinic in the coming decades [17]. Although several experimental types of research have been conducted to examine the potency of SCs on the management of bladder dysfunction, there is no general agreement on regenerative outcomes in empirical studies [18,19]. In the current systematic review, we highlighted the restoration of bladder function postSC transplantation in preclinical NGB dysfunction studies using meta-analysis on SCI and with a comprehensive systematic review in PD, stroke, and MS.
MATERIALS AND METHODS
Search Strategy
A systematic search was conducted in Embase, ProQuest, Cochrane library, Clinicaltrial.gov, WHO, Google Scholar, MEDLINE via PubMed, Ovid, Scopus, Web of Science, ongoing trials registers, and conference proceedings in June 2019 with no limit of date or language. The list of included review articles, experiments, and contacted authors of included trials were screened for subsequent analyses. We also monitored the abstracts from international congress. Unpublished or incomplete experiments were scoped via researchers known to participate in similar studies.
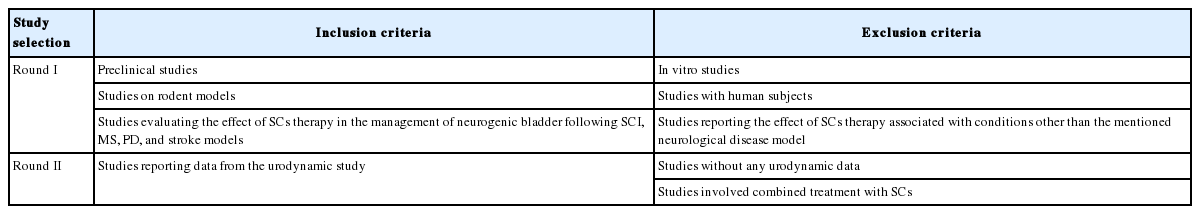
Inclusion and Exclusion Criteria
All preclinical studies about NGB and SC transplantation as therapy on rodents (e.g., mouse and rat) models of SCI, PD, MS, and stroke were included. We excluded all studies reporting the effect of SC therapy associated with conditions other than the mentioned neurological disease models or with human subjects or in vitro studies without any access to full-text. Inclusion and exclusion criteria are summarized in Table 1. Title and abstract screening process were performed independently by 2 investigators. Then, each author separately evaluated the full-text of selected articles. Any dispute among different study sections between reviewers was resolved by debate until unanimity was reached. A preliminary review of the data was done on the identified citations in study selection I. Due to the profound heterogeneity within the data from various studies, a wide range of point estimates was achieved and thereby a second study selection was done (study selection II) to screen most relevant results for further statistical analysis. All types of SCs or other progenitor cells were included in the current systematic review. Of note, autologous or human-derived bone marrow mesenchymal SCs (hBMSC), human amniotic fluid SC (hAFSC) and human umbilical cord blood SC (hUCMSC), oral mucosa, human embryonic SCs-medial ganglionic eminence (hESC-MGEs), human glial-restricted progenitors, immortalized neural SCs, neural-restricted progenitors (NRPs), glial-restricted progenitors (GRPs), human glial-restricted progenitors (hGRP) and astrocytes derived from hGRP, human-derived adipocyte SC, and interstitial cells of Cajal were enrolled in this study. The primary outcome measures were urodynamic-associated findings such as micturition pressure, residual urine volume, nonvoiding contraction, bladder capacity, micturition volume, maximum pressure, and detrusor hyperreflexia.
Data Extraction
Two authors independently recorded the information by using a data extraction form as follows: Author, year of publication and type, animal characteristics (including strain, species, and sex) and the model of neurological disease to induce NGB details, the characteristics of SCs (including type, route, time, dose, and frequency of transplantation), study quality evaluation, and the reporting of measures to reduce the risk of bias (see the following sections). We collected data for the nature of the outcome reported (urodynamic parameters), and animal number per group, mean outcome and standard deviation (SD) or standard error of the mean. In a single publication where various experiments were represented, the data were considered as independent experiments. Disagreement resolved in consultation with the third person. For graphically presented data, we monitored the values from graphs using Universal Desktop Ruler (ver. 2.9) or contacted the authors of the manuscript for the details.
Methodological Quality of Studies
For this propose, 2 reviewers assessed the methodological quality of the selected trials. The assessment of the risk of bias was done through a 6-criterion appraisal checklist containing sequence generation, allocation concealment, blinding, incomplete outcome data, and selective outcome reporting and other bias. The internal validity of the enrolled studies (e.g., selection, performance, detection, and attrition bias) and other study quality measures (e.g., reporting quality, power) were assessed using a modified version of the Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Studies (CAMARADES) quality checklist [15].
Statistical Analysis
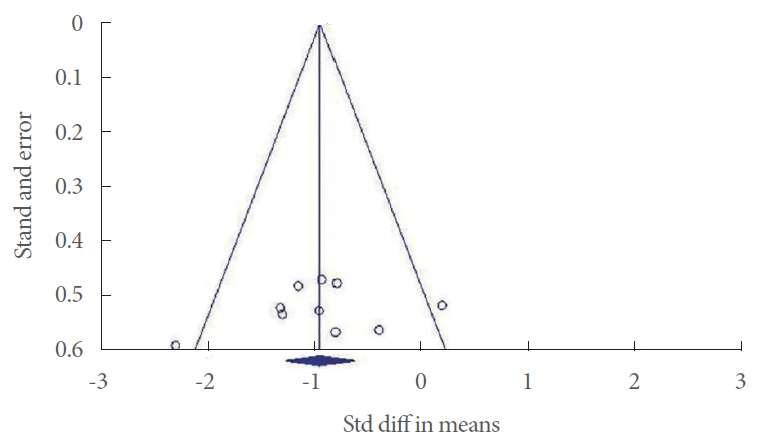
The outcomes of interest in the current analysis were the micturition pressure, micturition volume, maximum pressure, residual urine volume, nonvoiding contraction, or detrusor hyperreflexia in rodents with NGB induced by SCI. Results for PD, stroke, and MS were not subjected to the statistical analysis due to the lack of sufficient data. Meta-analysis of the data such as findings of urodynamic studies was done by using the MantelHaenszel method with Comprehensive Meta-Analysis software (ver. 2.2; Biostat, Englewood, NJ, USA). All variables were continuous data. Mean±SD was used to calculate the standardized mean difference and 95% confidence interval (CI). Statistical heterogeneity was analyzed using the I2 value and the result of the chi-square test. A P <0.05 and I2>50% were considered suggestive of statistical heterogeneity. We used the fixed model for low heterogeneity and mixed model for high heterogeneity in parameter analysis. Subgroup analysis was done whenever it was possible. Results of the comprehensive meta-analysis to examine any potential publication bias in the studies are shown as Funnel plots.
RESULTS
Description of Studies
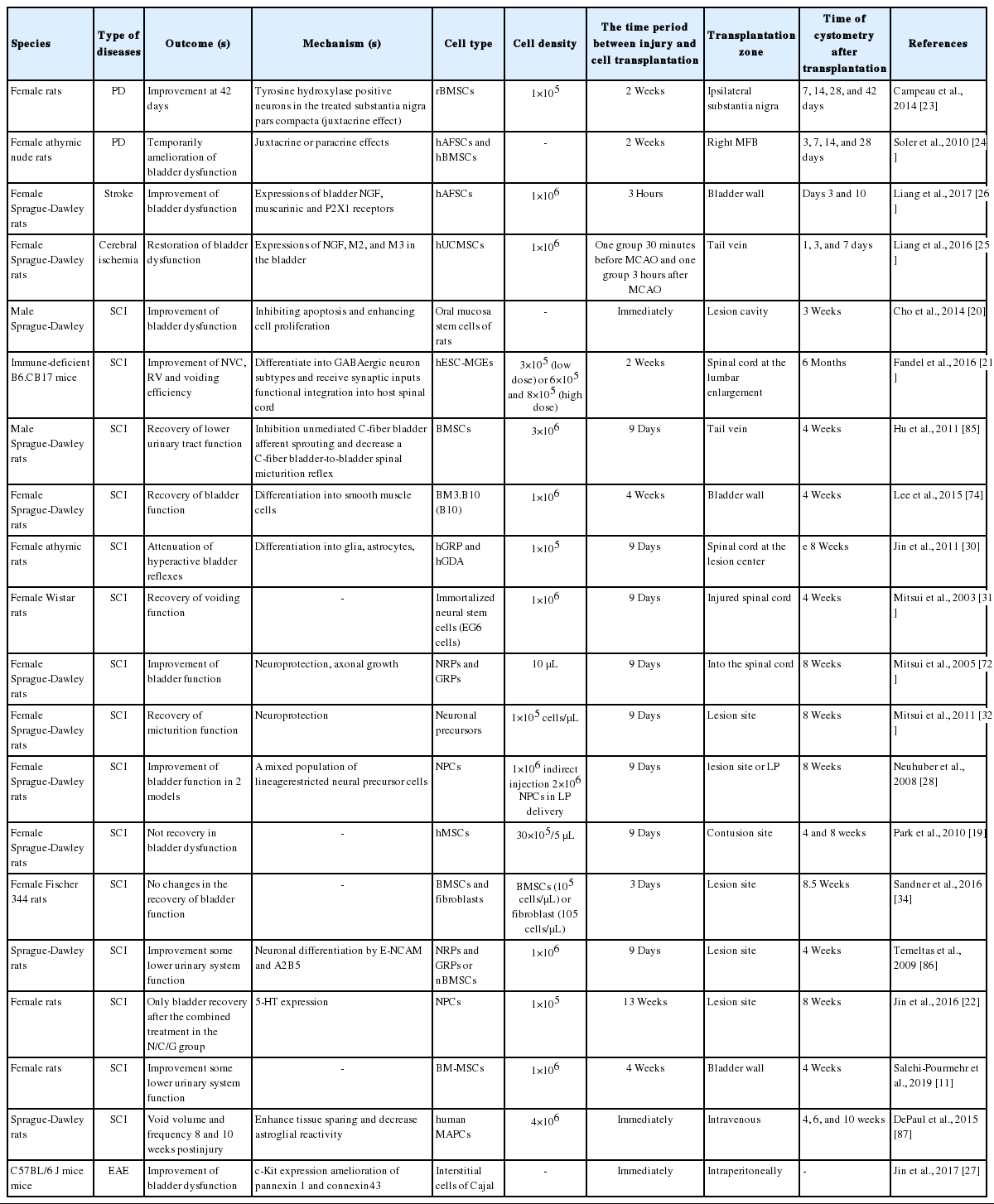
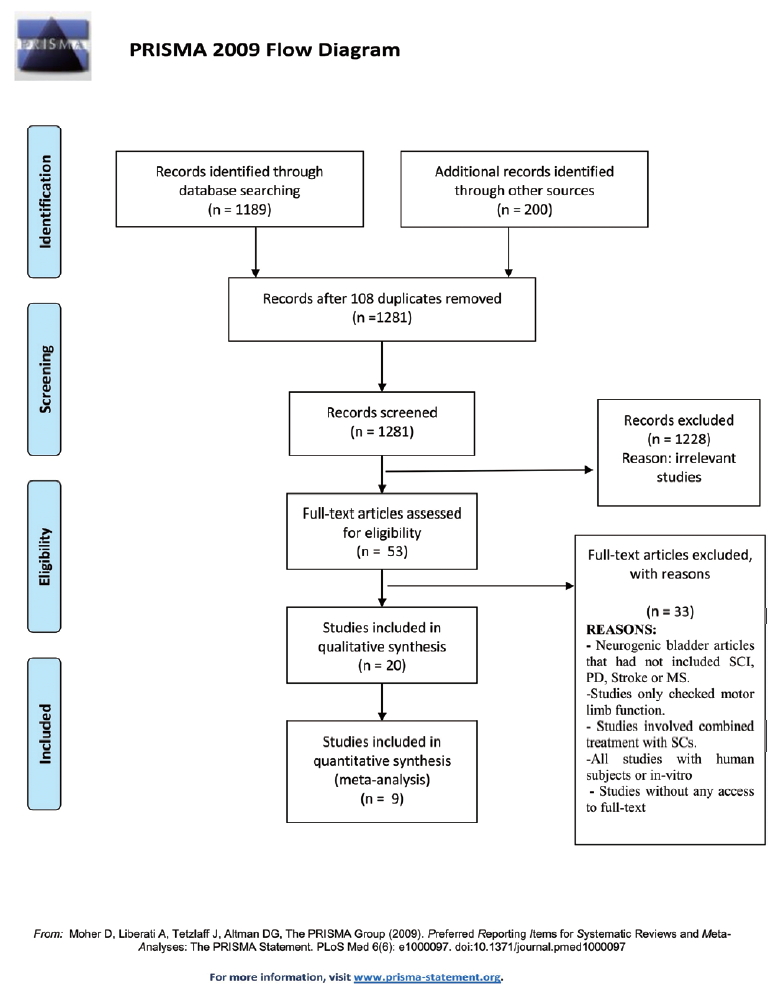
We found 1,189 relevant publications during the searching of electronic databases. Among them, 1,281 were excluded after an intensive screening of the titles and abstracts, duplicate publications, or human subjects. The full-text of 53 articles was evaluated and finally, 19 studies met our inclusion criteria that evaluated SC therapy effects on NGB (Table 2). Among the included studies, 15 publications were conducted on SCI (of these 3 were induced by transection model and others were contusion model), 2 on PD (6-hydroxydopamine administration into medial forebrain bundle region, 2 related to stroke and one correlated with MS rodent models. Most of the publications used rats and only 3 studies related to SCI and MS experiments used the mouse as a model. In 4 publications, male rats were examined while the others were conducted on female rats, and gender was not mentioned in 2 publications. The common SCs type used in the studies were neural progenitor cells (NPCs), BMSc, hAFSC, and hUCMSC. The common delivery routes for cell transplantation were intrathecal, intrabladder wall or intratail vein, substantia nigra, and medial forebrain bundle. In one of the SCI studies, urodynamic data were not in accordance with the other data thereby we didn’t include it in the meta-analysis [20]. Finally, 11 studies met all inclusion criteria. In one study, the urodynamic assessment was done 6 months post-cell transplantation [21] and in another study, NPC was transplanted 13 weeks after contusion lesion [22]. Therefore, we did not include these studies to analyze and eventually, 9 studies were used. A flow chart for data selection is represented in Fig. 1. We did not perform a meta-analysis in the other models of NGB (i.e., PD, stroke, and MS) due to the lack of sufficient studies.
Risk of Bias in the Included Studies
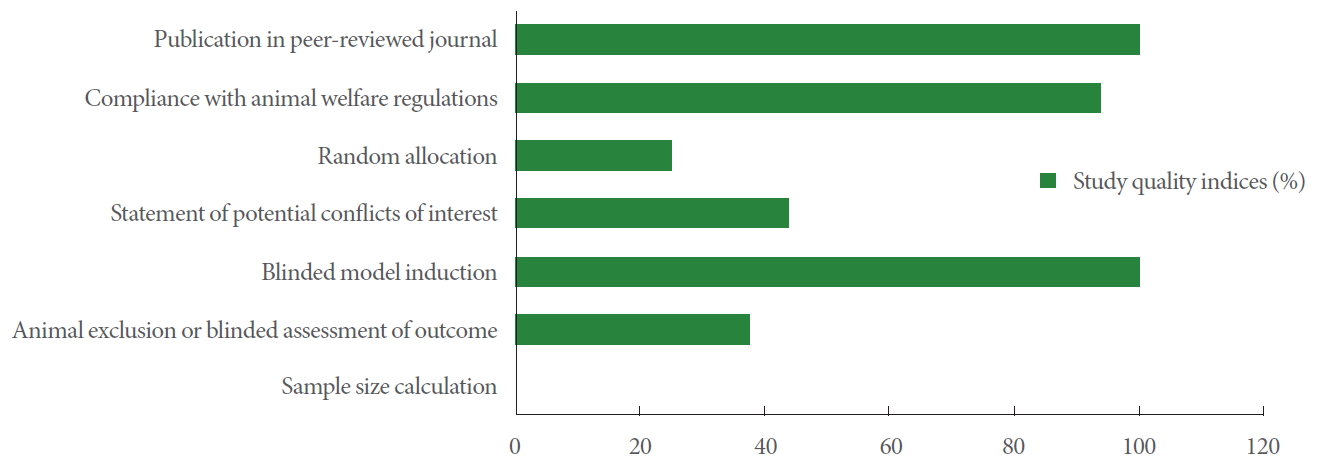
In the current study, a modified CAMARADES quality checklist was used to assess the internal and external validity of the selected studies. The checklist contains details notably randomized allocation (model/sham groups), blinded induction of the model and assessment of outcomes, calculation of the sample size, compliance with existing animal welfare act, the disclosure of all relevant conflicts of interest, reporting of animal exclusions, and publication in peer-reviewed journals. All articles had been issued in peer-reviewed journals. Seven studies had disclosure statements for conflicts of interest. All papers except one stated compliance with the animal protection act. The procedure of random assignment to the groups was seen in 25% of studies. All selected articles declared blinded induction of the animal model. No study existed to state the methodology of sample size calculation and only 6 studies illustrated both animal exclusion criteria and blind outcome assessment (Fig. 2).
Urodynamic Findings
Parkinson disease
Campeau showed that the ipsilateral transplantation of rat BMSCs (rBMSCs) into substantia nigra improved urodynamic parameters in the PD model up to 42 days. However, it was shown that encapsulated BMSCs (ErBMSCs) exacerbated the urodynamic parameters on days 7 and 14 after cell transplantation without any improvement during 42 days. rBMSC-treated animals showed a lower threshold, intermicturition pressure spontaneous activity, and uninhibited contraction 42 days post-cell transplantation compared to the control animals. The administration of ErBMSCs to rats yielded lower threshold pressure after 28 days and lower spontaneous activity on day 42 than that in rats given vehicle only [23]. In the study of Soler et al. [24], cystometric parameters were changed in all groups, 3 and 7 days after cell transplantation compared to parallel-matched controls. Cystometric parameters including bladder capacity, micturition pressure, spontaneous activity, and threshold pressure were improved in rats received BMSC and hAFSC 14 days after cell administration. On day 14, hBM-MSC injected rats showed improvement in micturition volume, bladder capacity, micturition pressure, mean bladder pressure between 2 micturitions, spontaneous activity, compliance, and threshold pressure. Moreover, nonsignificant differences were found in the cystometric parameter among the groups after 28 days [24].
Stroke
According to the study of Liang et al. [25], pre- and postadministration of SCs via the tail vein in ischemic rats, returned cystometric variables nearly to levels found in sham-operated rats. All rats were subjected to bilateral ovariectomy 2 weeks prior to middle cerebral artery occlusion (MCAO) to create a hypoestrogenic menopausal state. Results from cystometric analysis noted that ischemic rats showed increased values in peak voiding pressure and residual urine volume 1, 3, and 7 days after the induction of MCAO, which further decreased after cell administration. Both intercontraction intervals and voided volumes were reduced post-MCAO while these values increased after cell transplantation on days 3 and 7.
Liang et al. [25] stated that residual urine volume decreased significantly 10 days after treatment with the direct intrabladder transplantation of hAFSC compared to the sham and MCAO groups. Based on data, voided volume was increased 3 and 10 days after the induction of MCAO in the hAFSC-treated group. Also, intercontraction intervals were increased 10 days after cell transplantation compared to the MCAO group [26].
Multiple sclerosis
We found only one study evaluated the impacts of intraperitoneal injection with interstitial Cajal cells on bladder function. Marked bladder dysfunction was developed in experimental autoimmune encephalomyelitis (EAE) mice indicated by urinary retention, the increase of micturition, and urine output reduction per micturition. In mice treated with SC factor, the diameter of the bladder was reduced compared to the EAE mice [27].
Spinal cord injury
Among the remained studies that met our inclusion criteria in 2 stages of study selection, we only included the studies in our meta-analysis in which the time (28- or 56-day post-SCI induction) and route of cell transplantation (lesion site) were homogenous.
Micturition Pressure
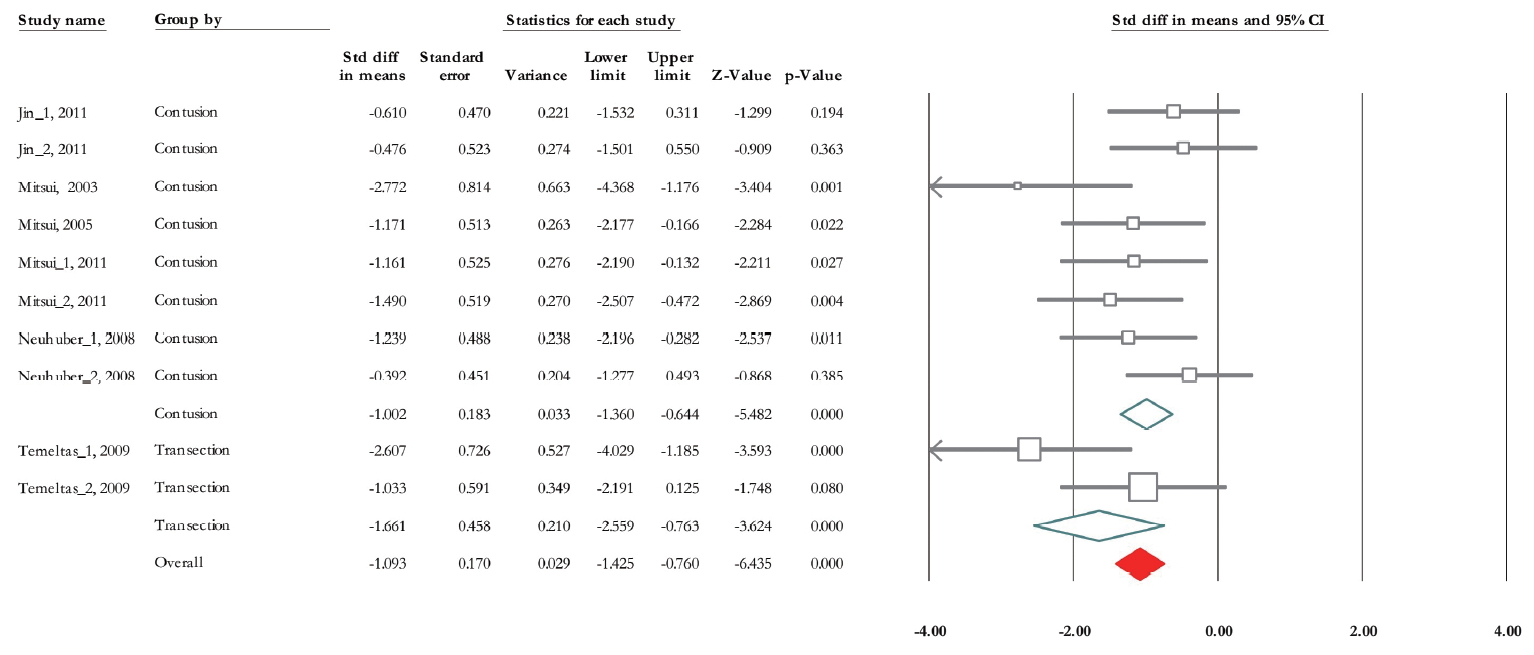
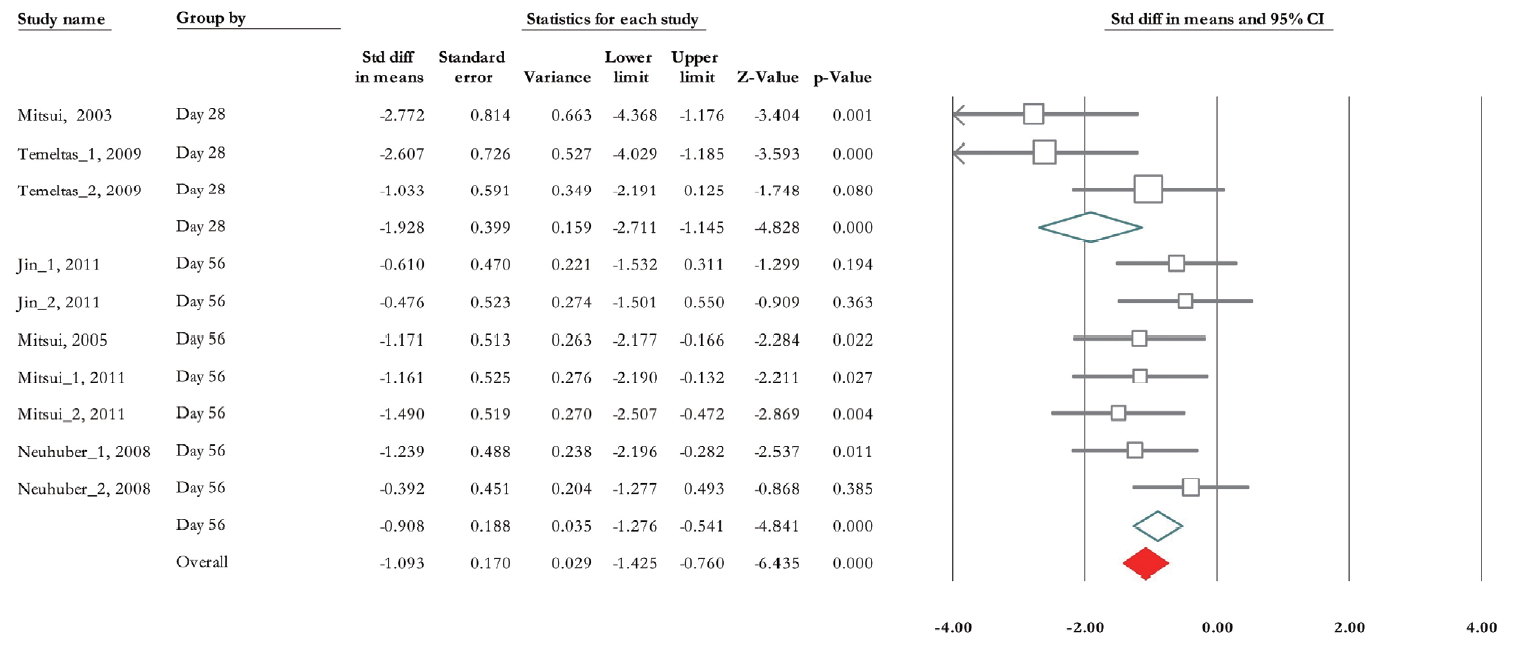
Of 10 trials, 6 experiments (n=155; 87 treatments and 68 nontreated controls) presented data correlated with micturition pressure [28-33]. The standardized mean difference (SMD) change of micturition pressure improvement from baseline was -1.093 (95% CI, -1.42 to -0.76) (P<0.001). Heterogeneity assay revealed P=0.116 and 36.5% of Higgins’ I2. In subgroup analysis, the SMD change of micturition pressure in the contusion model was -1.002 (95% CI, -1.36 to -0.64) (P<0.001) and in the transection model was -1.66 (95% CI, -2.56 to -0.76) (P<0.001) (Fig. 3). In the second subgroup study, the results of urodynamic showed that SMD change of micturition pressure 28 days posttransplantation reached -1.93 (95% CI, -2.71 to -1.15) (P<0.001) and it was -0.91 (95% CI, -1.28 to -0.54) (P<0.001) on day 56 (Fig. 4).
Residual Urine Volume
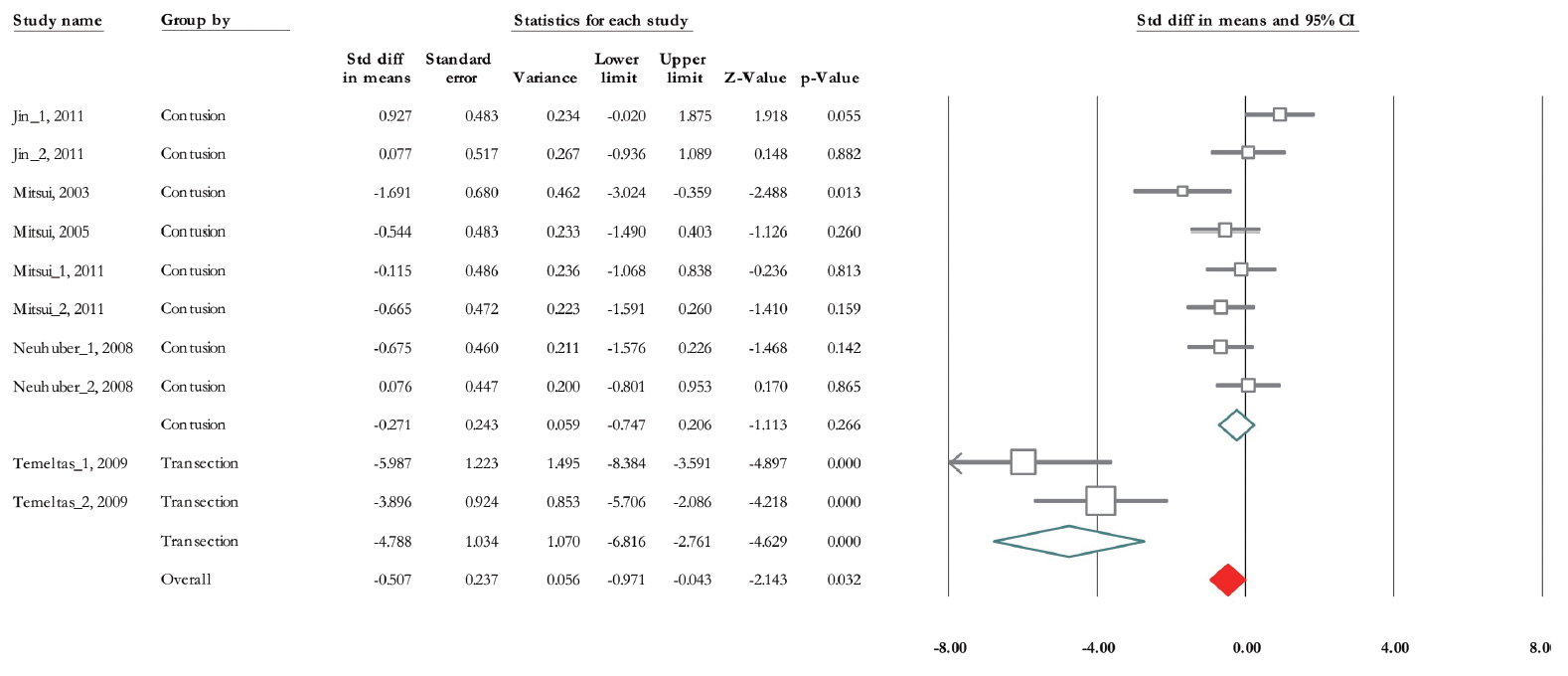
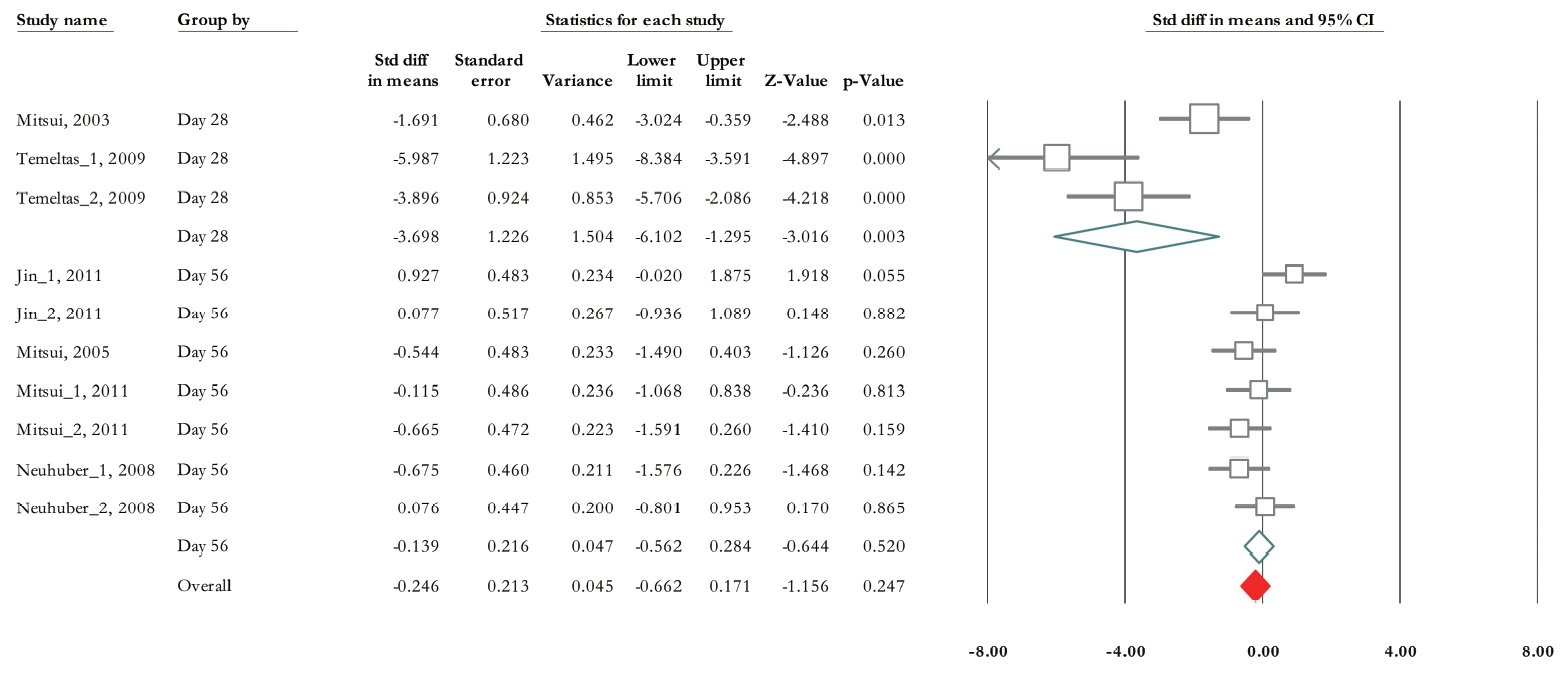
Of 10 trials, 6 experiments (n=155; 87 treatments and 68 non-treated controls) demonstrated data related to RV [28-33]. The SMD change of residual urine volume from basal levels based on fixed model analysis was -0.47 (95% CI, -0.81 to -0.14) (P=0.005) (Fig. 5). Heterogeneity assay revealed P<0.001 and Higgins’ I2 was 81.72%. However, after subgroup analysis based on SCI type, it decreased to 47.89% in contusion and 46.31% in transection model. Also, mixed model subgroup analysis results showed that in contusion model, the SMD was -0.27 (95% CI, -0.74, to 0.2) (P=0.266) while it was -4.78 in transaction model (95% CI, -6.81 to -2.76) (P<0.001) (Fig. 5). Also, we performed a subgroup analysis based on urodynamic date post-cell transplantation. Twenty-eight days after treatment, SMD was -3.69 (95% CI, -6.10 to -1.29) (P =0.003) and in 56 days post-cell transplantation was -0.13 (95% CI, -0.56 to 0.28) (P=0.52) (Fig. 6). Heterogeneity did not differ in subgroup analysis in 28 days after cell transplantation but it was reduced after 8 weeks postcell transplantation (Higgins’ I2=30.05%, P=0.199).
Nonvoiding Contraction
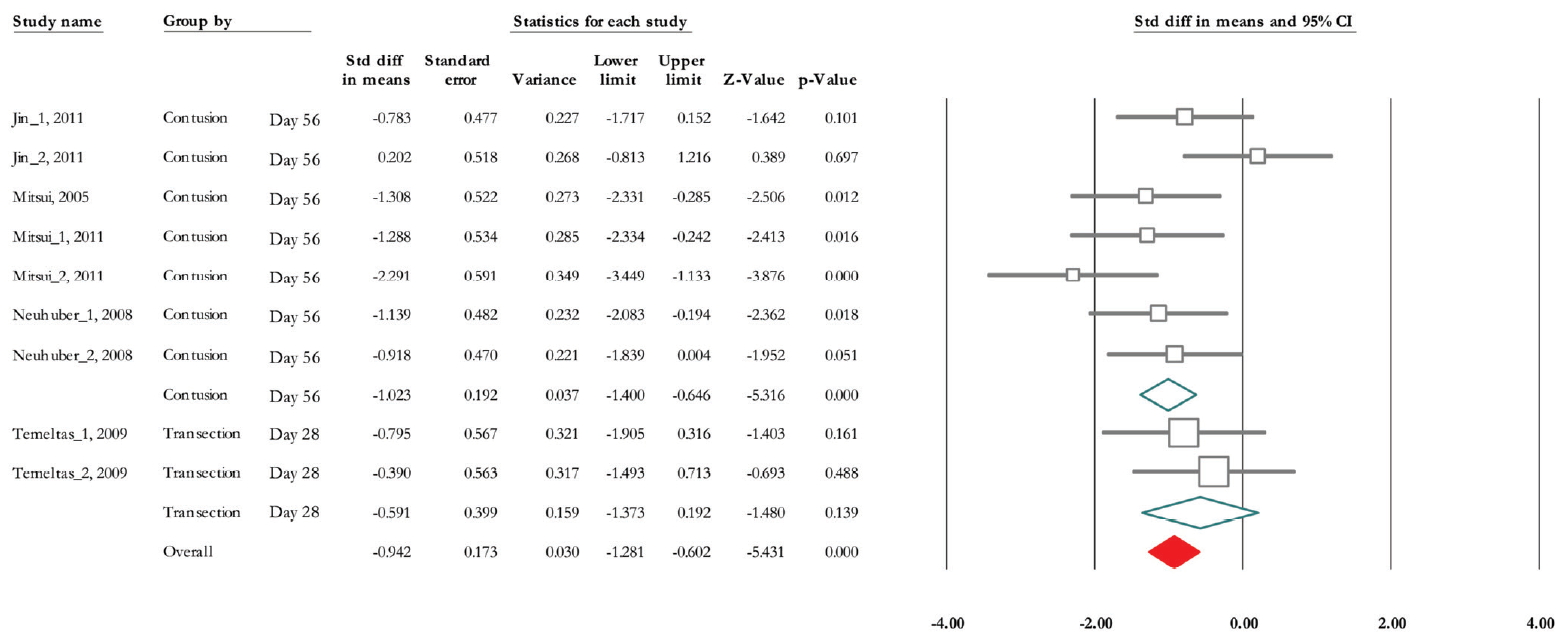
Of 9 trials, 5 experiments (n=143; 80 treatments and 63 controls) released data on nonvoiding contraction [28-30,32,33]. The SMD change of nonvoiding contraction alleviation was -0.942 (95% CI, -1.281 to -0.602) (P <0.001). Heterogeneity analysis indicated P=0.138 and Higgins’ I2 was 35.03%. Subgroup analysis results showed that in contusion model and 56 days after cell implantation, SMD was -1.023 (95% CI, -1.40 to -0.65) (P<0.001) and in transaction model and 28 days after treatment it was -0.591 (95% CI, -1.37 to 0.19) (P=0.139) (Fig. 7).
Bladder Capacity
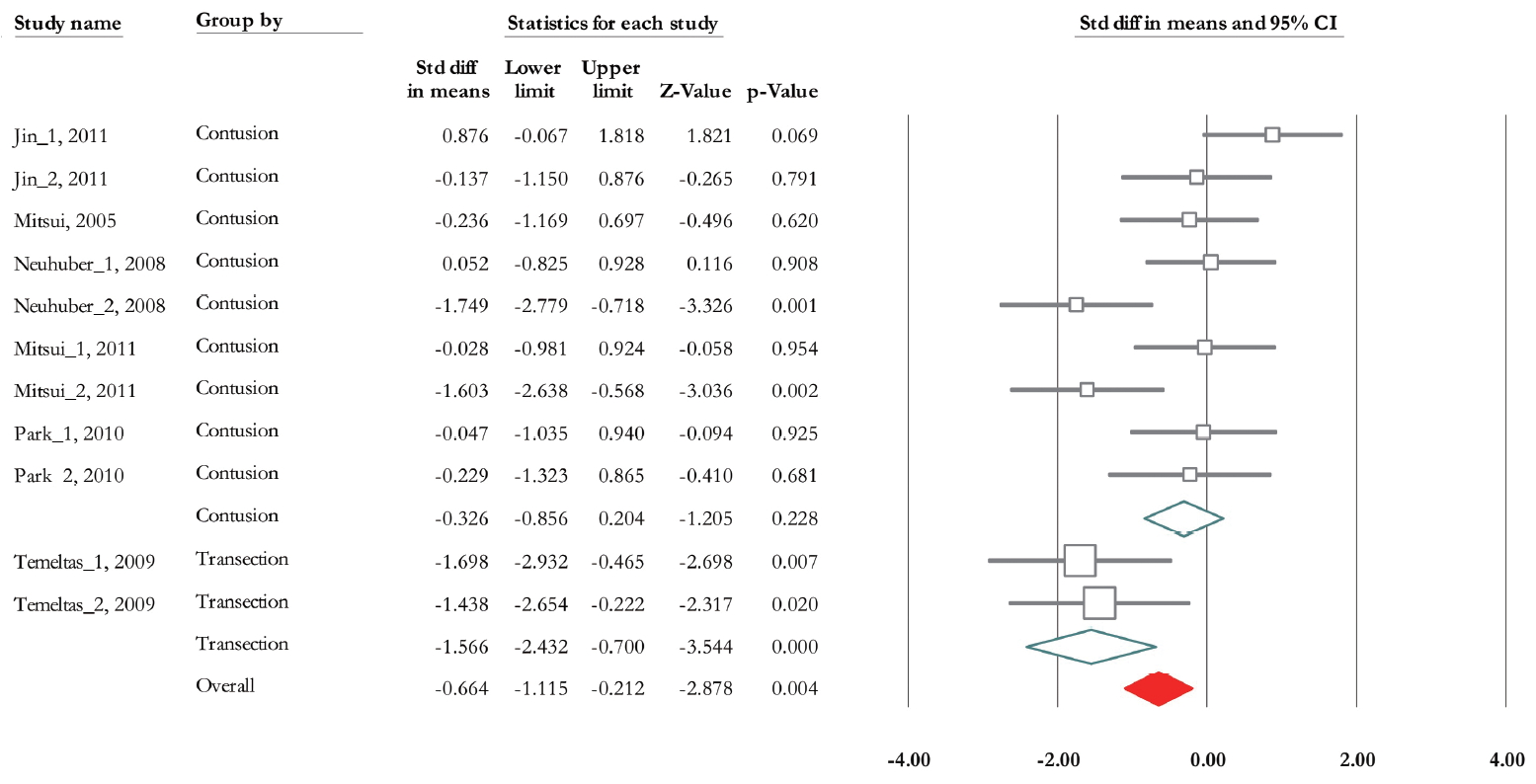
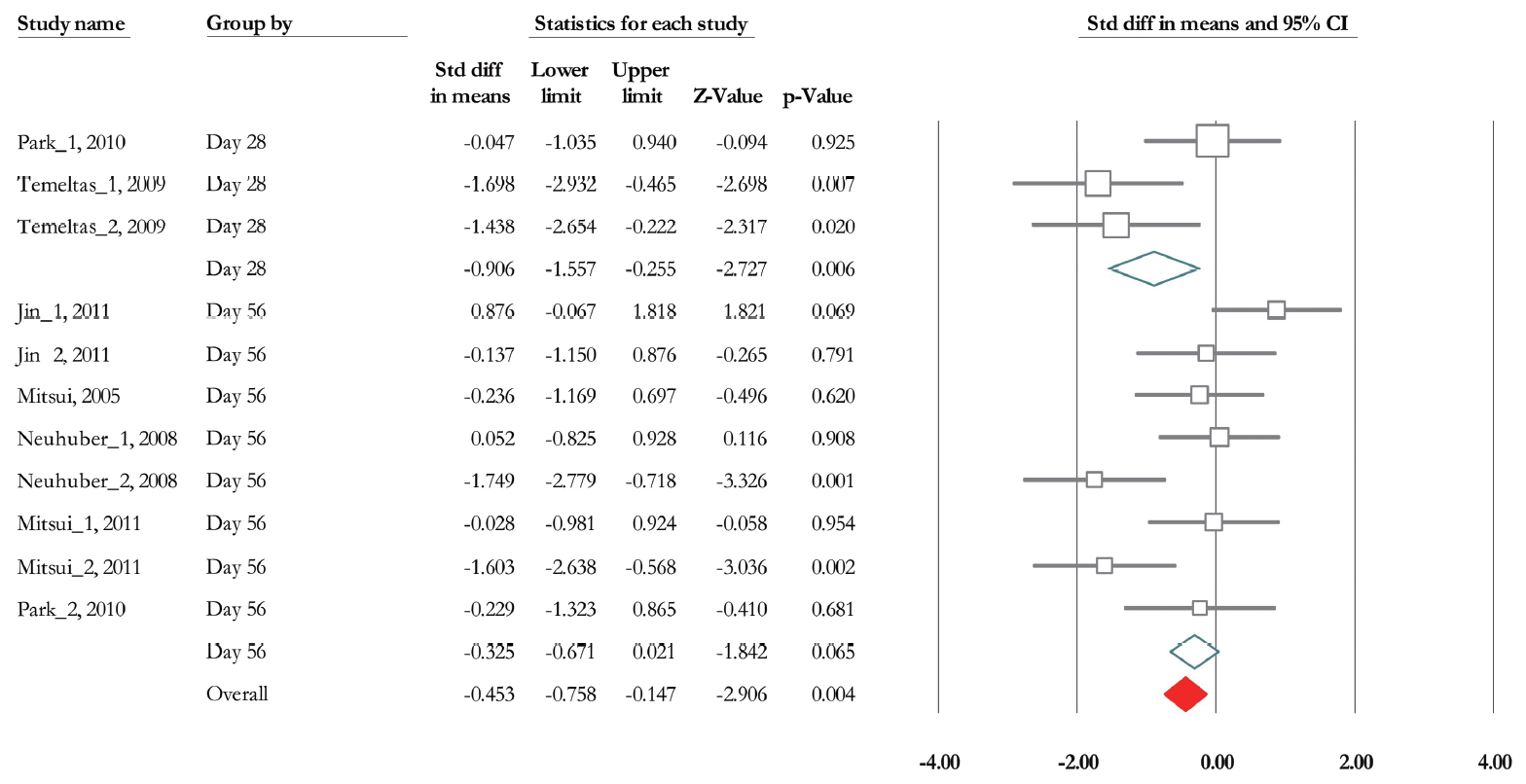
Of 11 trials, 6 experiments (n=185; 93 treatments and 92 controls) released data related to bladder capacity [19,28-30,32,33]. Overall SMD based on fixed model analysis was -0.453 (95% CI, -0.758 to -0.147) (P =0.004). Heterogeneity analysis revealed P=0.002 and Higgins’ I2 was 64.67%. The SMD change of bladder capacity based on mixed model analysis in contusion and transaction model was -0.32 (95% CI, -0.85 to 0.2) (P=0.228), and -1.566 (95% CI, -2.43 to -0.70) (P<0.001) (Fig. 8). Also, it was -0.906 (95% CI, -1.56 to -0.26) (P=0.006), and -0.325 (95% CI, -0.67 to 0.021) (P=0.065) at 28 and 56 days after transplantation (Fig. 9). Heterogeneity did not decrease in subgroup analysis based on urodynamics date, however in transection model Higgins’ I2 was 0% (P=0.768).
Micturition Volume
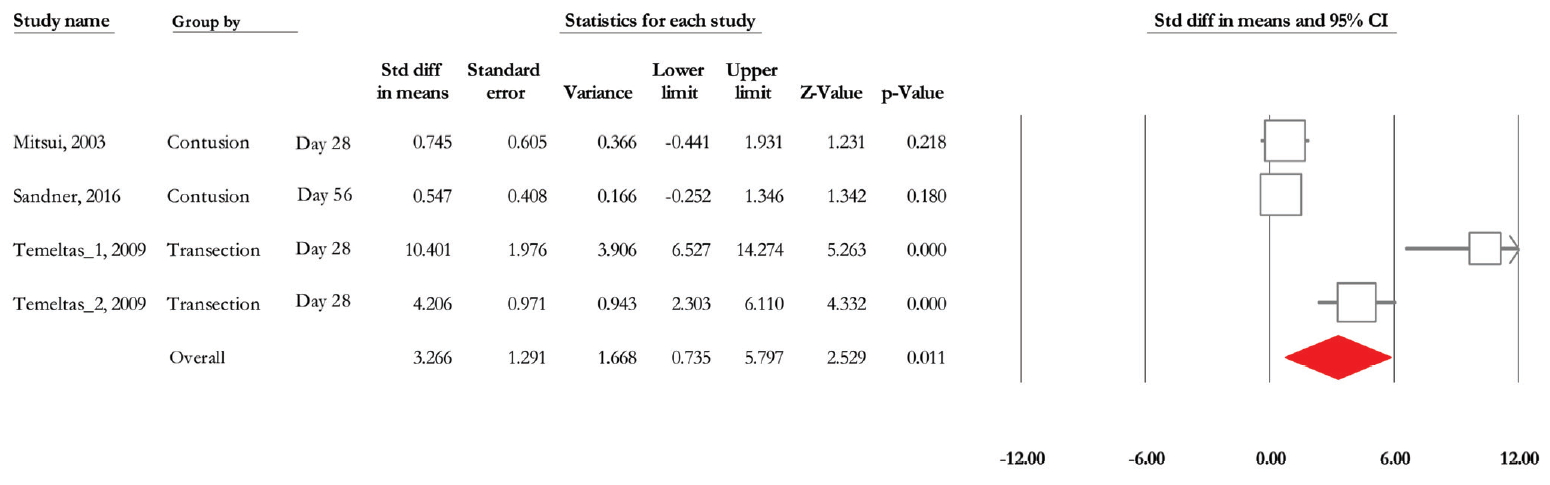
Of 4 trials, three experiments (n =66; 38 treatments and 28 controls) released data on micturition volume [29,31,34]. According to the mixed model analysis, the SMD change of micturition volume reached 1.23 (95% CI, 0.619 to 1.855) (P < 0.001) (Fig. 10). Heterogeneity analysis revealed P<0.001 and Higgins’ I2 was 91.27%. Due to the limited number of experiments related to micturition volume, we could not conduct subgroup analysis.
DISCUSSION
Laboratory animals are widely used to evaluate the medication’s efficacy and toxicity. The biological similarity to humans is one of the most important characteristics of laboratory animals such as mice and rats [35]. Thus, in most experimental NGB studies, these animals are preferred. The regulation of micturition requires connections between the brain and extensive tracts in the spinal cord that involve sympathetic, parasympathetic, and somatic systems. Damage of the cerebral cortex, brain stem, basal ganglia, and spinal cord following neurological disorders lead to NGB [36-40]. Generally, upper motor neuron lesions (above the sacral cord) can lead to neurogenic detrusor overactivity and underactive or acontractile detrusor could be evident after lower motor neuron injuries such as the lower lumbar cord, conus or cauda equina lesions and peripheral neuropathies [41,42].
NGB Following Stroke
MCAO to induce an acute ischemic model [43] and common carotid occlusion induced ischemia are 2 common animal models that lead to bladder overactivity. The variety of receptors including dopamine, glutamate, and gamma-aminobutyric acid (GABA) receptors are involved in the pathogenesis of these models [44-46]. It has been shown that reduction of nerve growth factor, as well as increment of muscarinic receptors in central and peripheral nervous systems, play a role in the regulation of the micturition reflex [43,44,47]. Nerve growth factor mediates morphological and functional changes in sensory neurons innervating the bladder [47], and muscarinic receptors contribute to control of the micturition reflex [46]. Following a hemispheric stroke, urinary complaints such as nocturnal urinary frequency, urinary incontinence, difficulty in voiding, and urinary retention are common. Also, NGB may lead to a considerable alteration in cystometric parameters. For example, in the hemispheric stroke, urodynamic study results show detrusor overactivity, a condition is related to uncontrolled relaxation sphincter and nonrelaxing urethra [48] and in cerebral hemorrhage moreover, the detrusor overactivity, low-compliance bladder, acontractile detrusor and detrusor-sphincter dyssynergia is seen [49].
NGB Following PD
Bladder dysfunction is a common nonmotor complication in PD. The brain pathology causing the bladder dysfunction involves an altered dopamine in basal ganglia-frontal circuit, which suppresses the micturition reflex [50]. This reflex is under control of dopamine [51] (D1: inhibitory and D2: facilitatory) and GABA (inhibitory).
The loss of dopaminergic neurons in the substantia nigra, which is usually achieved by injecting 6-hydroxydopamine in medial forebrain of rats for modeling of PD [52], is associated with reduction of striatal dopamine levels. This causes a reduction of the D1 output and leading to detrusor overactivity [53]. The most common urodynamic study finding in PD is detrusor overactivity [54,55].
NGB Following MS
EAE is the most common animal model in a rodent for the induction of MS-like pathology. Demyelination in this model is associated with the infiltration of T cells, macrophages, and B cells [56]. Active induction by immunization with myelin antigens and passive induction by the adoptive transfer of preactivated myelin-specific T cells into naïve mice are 2 main methods of EAE induction in the mice [57,58]. Myelin oligodendrocyte glycoprotein peptide (MOG35–55), which induces relapsing-remitting EAE in C57BL6 mice has been confirmed to be a useful model to investigate the mechanisms of NGB [59]. Urodynamic findings of the EAE model are included detrusor-external sphincter dyssynergia, detrusor overactivity, and detrusor hypocontractility [60,61].
NGB Following SCI
Several SCI induction models are reported in the literature [62]. Spinal cord transection and contusion models are frequently used methods for the induction of NGB [63].
SCI causes severe deficits in the urinary system besides of sensory and motor loss [37]. Urinary dysfunction is the result of supraspinal input, afferent input to the spinal cord disruption, and reorganization of intraspinal circuitry in response to injury [38]. Detrusor-sphincter dyssynergia, hyperreflexia, and autonomic dysreflexia are the result of this disruption, too [39]. Additionally, significant structural, physiological, and molecular alterations occur in the bladder in SCI conditions [40]. In spinal shock phase of SCI, the bladder is acontractile. Although catheterization controls this disruption in human, it decreases the quality of life and increases the rate of urinary tract infection. Tissue destruction of the pontine micturition centers located at the sacral cord shows the synergy removal between the detrusor and sphincter, contributing to detrusor-sphincter dyssynergia [64,65]. Complete deterioration of bladder compliance, function, infection, and other lower urinary tract complications are the other consequences of SCI [66] and also increment of bladder wall thickness as a result of larger collagen percentage is a common sequel of NGB induced by SCI [67]. Considering the underlying recovery mechanism, it was revealed, the type C fibers are the major afferents in a spinal segmental reflex [41]. In the case of NGB dysfunction, the sensitization of C-fiber afferent bladder, and destroying of GABAergic input to preganglionic neurons of parasympathetic lead to nonvoiding contraction in the bladder detrusor muscles [68]. Following SCI, disruption of neuron entry from supraspinal micturition center impairs the coordination among the detrusor and external urethral sphincter [69].
SCs transplantation in the management of neuro-urological diseases is accompanied with promising outcomes. However, its application is limited in the clinic. Three major types of SCs e.g. adult SC or tissue-specific SC, embryonic stem cells, and induced pluripotent stem cells [70] are more frequently used cells in the experimental and clinical researches. The ability of myelin regeneration and axonal growth promotion and, differentiation of SCs into neuronal or glial cells are the major reasons for selection the specific type of SCs. Despite the limitations and difficulties, conducting researches on the recovery of bladder is a promising approach in the field of neuro-urology. In the studies included in this review, the frequently used cells were bone BMSc, hAFSC, hUCMSC, and NPCs. The viability of NRP/GRP cells in the injured spinal cord and the ability of migration, differentiation and neuronal development of these cells are reported in the previous studies [71]. Also, different studies reported that development in the recovery of the bladder function was achieved by transplantation of NPC [29,33,72]. SCs are biologically self-renewing adult cells with the potency of differentiation to many types of cells even different tissues. Also, these cells can be used in the recovery of the damaged bladder by differentiation into smooth muscle cells of the bladder [73,74]. Mechanisms of SCs involved in the recovery of bladder dysfunction are include migration, differentiation, and paracrine effects [73] or in PD model, neurons with tyrosine hydroxylase activity in the treated substantia nigra pars compacta, suggesting that functional improvement requires a juxtacrine effect [23].
However, there are some concerns about the use of NPCs including the lack of inefficient tracking systems, moderate cell survival [18,75], and glial scar formation [76]. Among the enrolled studies, most cells used in the SCI model were NPCs. Irrespective of its cause, after SCI, disruption of neural circuits influences excitatory and inhibitory inputs and result in failure of special cellular functions in neurons [77]. The ability of NPCs in neuronal regeneration following direct transplantation into the injured site is the main reason for using these cells. The other frequently used cell was MSCs that are examined in clinical trials too [78-81]. Its safety is approved [82] and the ability of transdifferentiation into neurons and glial cells is shown [83,84]. Previously, a meta-analysis study evaluated the bladder recovery by SC therapy in SCI-induced bladder dysfunction with controlling the SCI model to reduce heterogeneities [18]. In our systematic review and meta-analysis, in addition to the controlling SCI model (transaction or contusion), the timing of the urodynamic assessment (28 days or 8 weeks post-cell transplantation) also was evaluated as a possible source of heterogeneity. The other important factors that may be influenced on the outcome of the study were the used cell type, the route, and timing of cell transplantation. In the majority of the included studies in the meta-analysis, the rout of transplantation was direct injection into the lesion site or intrathecal. We did not include the other rout of transplantation in our analysis. Also, the timing of cell transplantation was similar and 9-day postinjury. Therefore, we did not perform the subgroup analysis for these issues. The other important factor was the rout of transplantation. The main rout in SCI model was intrathecal and the other less common methods were an intrabladder wall and/or intravenous. Hence, due to this heterogeneity, we only included only intrathecal administration in our meta-analysis. The cell type did not consider as a heterogeneous factor, due to the cells entity was the same and they possess neuro-regeneration capacity. Moreover, we enrolled the other neurological diseases that may be a cause of NGB.
In conclusion, the present systematic review and meta-analysis regarding bladder recovery after SC therapy in SCI demonstrate significant improvements of pressure after SC transplantation in both contusion and transaction models in 2-time points i.e., 4 and 8 weeks. Residual urine volume, micturition volume, and bladder capacity improved after treatment only in the 28 days after transplantation in the transaction model of SCI. Nonvoiding contraction recovered only in 56 days posttransplantation in the contusion model. Considering the application of SC in SCI preclinical studies, high-quality experiments to reduce the potential risk of bias are necessary for improved understanding of bladder recovery. Also due to limited studies on the relation of other neurological disorders such as PD, stroke, and MS with NGB, additional studies with the modified methodology to reduce the risk of bias are needed to prove the underlying mechanism and to achieve an appropriate approach for bladder recovery.
Notes
Fund/Grant Support
This study was financially supported by Research center for Evidence-Based Medicine Tabriz University of Medical Sciences (grant No. 59224).
Research Ethics
This meta-analysis was approved by the local ethical committee of Tabriz University of Medical Sciences (IR.TBZMED. REC.1397.451).
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTION STATEMENT
·Conceptualization: HSP, SH, RR, JM, NM
·Data curation: HSP, FP, JM, NM
·Formal analysis: SSE
·Funding acquisition: SH
·Methodology: SH, FP, SSE
·Project administration HSP, SH