Association Between Overactive Bladder and Polyneuropathy in Diabetic Patients
Article information
Abstract
Purpose
Diabetes mellitus (DM) is a chronic metabolic disorder that often leads to complications. We aimed to correlate two complications of DM, polyneuropathy and hyperactive bladder syndrome, using noninvasive measures, such as screening tests.
Methods
We included 80 female and 40 male type 2 diabetic patients in this prospective study. Diabetic polyneuropathy evaluations were conducted using the Douleur Neuropathique 4 Questions (DN4), and overactive bladder (OAB) evaluations were performed using the Overactive Bladder Questionnaire (OAB-V8). The patients were also evaluated for retinopathy and nephropathy. The diabetic male and female patients with or without OAB were chosen and compared for microvascular complications (polyneuropathy, retinopathy, and nephropathy).
Results
There were no significant correlations between OAB and retinopathy as well as between OAB and nephropathy among diabetic patients (female patients, P>0.05; male patients, P>0.05). However, the patients with OAB were significantly more likely to develop polyneuropathy (female patients, P<0.05; male patients, P<0.05).
Conclusions
In diabetic patients, OAB and diabetic peripheral neuropathy are significantly correlated. These correlations were demonstrated using short, understandable, valid, and reliable disease-specific tests without invasive measures. Using these screening tests, both neurologists and urologists can easily diagnose these complications.
INTRODUCTION
Diabetes mellitus (DM) is a chronic disease that can lead to many complications, including peripheral sensorimotor polyneuropathy, which is very common [1]. The distal lower extremities are predominantly affected. If the disease progresses, sensory loss proceeds proximally, eventually affecting the hands. The incidence of peripheral neuropathy was reported at 5%–71% [2].
Diabetic bladder dysfunction (DBD), which commonly accompanies DM. The most common defined bladder dysfunction is detrusor overactivity (DO) with and without impaired detrusor contractility. Its prevalence is reported at 25%–87% [3]. Studies have shown that overactive bladder (OAB) disorder is associated with storage symptoms (e.g., urinary urgency with or without urinary incontinence, usually with increased urinary frequency and nocturia) [4-6]. OAB affects 39%–61% of diabetic patients [7,8].
Correlation between bladder dysfunction and the peripheral nervous system has been demonstrated in many studies [9-11]. These studies used urodynamic tests to diagnose DBD and neurophysiological tests to diagnose diabetic polyneuropathy. We used the Douleur Neuropathique 4 Questions (DN4) and the Overactive Bladder Questionnaire (OAB-V8) to identify the correlations between diabetic polyneuropathy and OAB. We aimed to determine the correlations between these conditions using noninvasive, short, easily applicable, valid, and reliable tests.
MATERIALS ANS METHODS
Study Population
This prospective study included 80 female and 40 male patients for a total of 120 patients with type 2 DM. The patients with benign prostatic hyperplasia, malignancies, urinary tract infections, a history of major pelvic surgery, pelvic organ prolapse, major neurologic diseases (e.g., Parkinson, Alzheimer, or cerebrovascular disease), chronic renal or hepatic disease, and vitamin B12 deficiency, as well as those taking diuretics were excluded from the study. DM was diagnosed according to the American Diabetes Association Criteria [12]. According to the World Health Organization classification, the body mass indices (BMIs) were calculated using the patients’ height and weight measurements [13]. Routine hematological and biochemical analyses were performed on venous blood samples between 9:00 AM and 10:00 AM after an overnight fast.
This work was approved by the Ethics Committee of Bozok University, Medicine Faculty (13.03.2015/79), and all patients provided written informed consent.
Assessment of Peripheral Neuropathy
To diagnose polyneuropathy, the DN4 was used (Supplementary material). The DN4 is a clinician-administered questionnaire containing 7 items related to symptoms and 3 items related to neuropathic pain examination. The total score is calculated as the sum of the 10 items, and a total score of 4 or more suggests neuropathic pain. The DN4 showed 83% sensitivity and 90% specificity in the diagnosis of neuropathic pain in a developmental study [14]. The DN4 Turkish version has been validated [15].
Assessment of OAB
For the diagnosis of OAB, the OAB-V8 was applied. This commonly used form consists of eight questions with a total score of 40. The Turkish version was validated and subjects with 11 points or higher was accepted as OAB [16].
Assessment of Retinopathy
All patients underwent ocular examination by the same ophthalmologist. Retinopathy was defined as the presence of characteristic changes, including hemorrhages, exudates, laser marks, and fibrous proliferation, detected by ophthalmoscopy through dilated pupils. Diabetic retinopathy is classified as either nonproliferative or proliferative diabetic retinopathy [17].
Assessment of Nephropathy
The estimated glomerular filtration rate was calculated using the Cockcroft-Gault equation. The albumin secretion rate was calculated using the albumin: creatine ratio in the urine. Microalbuminuria (≥30 mg) and macroalbuminuria (>300 mg) were diagnosed based on the urinary albumin:creatinine ratio [18].
Statistical Analysis
The SPSS ver. 16 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. The age, duration of diabetes, BMI, routine hematological and biochemical analyses were reported as the mean±standard error. The independent sample t-test was used to compare the differences between continuous variables, while Chi-square analysis was used to assess the differences between categorical variables. P-values <0.05 were considered to indicate statistical significance.
RESULTS
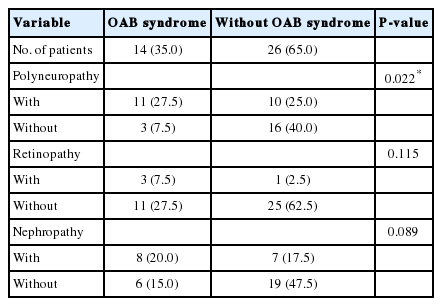
The demographic and laboratory data of all diabetic patients are shown in Table 1. Diabetic female patients with and without OAB were compared according to microvascular complications (polyneuropathy, retinopathy, and nephropathy) (Table 2). There were no significant correlations between OAB and retinopathy as well as between OAB and nephropathy among female patients (P>0.05). There was a significant correlation between OAB and polyneuropathy among female patients (P<0.05). Male patients with and without OAB were compared according to microvascular complications (polyneuropathy, retinopathy, and nephropathy). There were no significant correlations between OAB and retinopathy as well as between OAB and nephropathy among male patients (P>0.05). There was a significant correlation between OAB and polyneuropathy among male patients (P<0.05) (Table 3).

Comparisons of microvascular complications between diabetic women with and without overactive bladder (OAB) syndrome (n=80)
DISCUSSION
This study revealed two significant findings. First, there is a significant correlation between OAB and diabetic peripheral neuropathy among diabetic patients. More importantly, this correlation was shown using noninvasive, short, and technically simple screening tests. Second, this correlation was not found in other microvascular complications of diabetes, such as retinopathy and nephropathy.
Most cases of diabetic neuropathy present in a distally symmetrical form due to progressive axonopathy.
Sensory symptoms precede motor symptoms in diabetic polyneuropathy. The function of small fibers (C-fibers without myelin and A-gamma with thin myelin) is affected in the early stages of DM. Large fibers (thick myelinated A-alpha and A-beta) are not affected [19]. No standard diagnostic criteria exist for small fiber neuropathy. Standard nerve conduction studies involving electromyography (EMG) do not help diagnose small fiber neuropathy. A neurophysiologic test that records sympathetic skin responses is sometimes used, but the responses are unreliable and the sensitivity is low for small fiber neuropathy [20]. Quantitative sensation tests (vibration, hot-cold, and thermal pain level) require patient cooperation, and an abnormal test result does not necessarily indicate a peripheral nervous system disorder, as any nervous system disorder can affect the results [21,22]. Thus, the tests used to diagnose small fiber neuropathy have only limited applicability.
Many studies have shown that axonopathy and small fiber neuropathy may take part in the early stages of diabetic neuropathy and OAB. Another study showed that axonopathy may cause DBD [23]. In these patients, axonal swelling and degeneration were seen [24]. Other studies have identified defects in A-gamma and C small fiber bladder afferent neural pathways [25,26]. All these findings confirm small fiber autonomic neuropathy in the bladder [27]. Small fiber autonomic neuropathy may increase smooth muscle contractility and detrusor muscle overactivity [25,26]. The presentation of peripheral neuropathy is hypersensitvity, hyposensitivity, and anesthesia. It has been proposed that the same type of pathology may lead to OAB [19]. A study in female patients with OAB and diabetes showed DO following bladder oversensitivity [28]. Invasive urodynamic studies, including those involving cystometry, have been used to diagnose bladder dysfunction. Unfortunately, cystometry may not show the pathological changes associated with OAB in up to 40% of patients with the condition [29]. Another urodynamic study tool is the uroflowmeter, which can differentiate true obstruction from weak detrusor contractility [30]. For the most part, techniques used for the diagnosis of bladder dysfunction and small fiber neuropathy have limited efficiency, and an accepted set of diagnostic criteria has yet to be formulated.
In a study of 94 diabetic female patients, OAB was diagnosed in 36.2% [28]. The incidence of OAB among female patients reported in this study (32.5%) is similar to that mentioned in the previous study and is consistent with that observed in males (35%). Lee et al. [9] performed a study that included 137 female diabetic patients who were followed up for 11 years. The authors examined the early stages of autonomic DBD and distal symmetric diabetic neuropathy, and diagnosed polyneuropathy in 67.2%, compared to our finding of 61.3%. Lee et al. [9] used the current perception threshold test for C-fiber functions to diagnose diabetic polyneuropathy. Uroflowmetry, postvoid residual urine volume, and the OAB symptom score questionnaire were also used. They concluded that C-fiber dysfunction in the distal extremities of female patients may be a marker of early DBD. Bansal et al. [10] studied 52 diabetic male patients with lower urinary tract symptoms (LUTS) and attempted to diagnose combined neuropathy using urodynamic studies, hand and foot sympathetic skin responses, and motor and sensory nerve-conduction velocity studies. They found the condition in 51.9% of male diabetic patients, compared to our finding of 52.5%. Therefore, male patients with DM and LUTS are likely to have neuropathy, which, in turn, may probably be associated with DBD. Another study of 29 diabetic patients used peroneal and sural nerve conduction studies and urodynamic and denervation supersensitivity tests and found meaningful correlations with vesicourethral dysfunction and peripheral neuropathy [11]. Neuropathy was found in 69% of patients with voiding dysfunction. We found polyneuropathy in 84.6% of female patients and 78.6% of male patients with voiding dysfunction. The polyneuropathy ratio found in this study was higher than that of the mentioned study, which may be due to the failure to detect small fiber neuropathy using EMG. In a previous study of 153 type 2 diabetic patients with LUTS symptoms, sensory thresholds (vibratory, thermal, and touch) and uroflowmetry were used. That study concluded that neuropathy is a predictor of bladder dysfunction [31]. In our study, we found OAB in 44.9% of female patients and 52.4% of male patients with polyneuropathy. These findings support those of the mentioned study.
Our study’s results regarding the relationship between diabetic polyneuropathy and OAB using screening tests were similar to those that used urodynamic and neurophysiologic tests, which supported the reliability of the screening tests. We used DN4 to diagnose diabetic polyneuropathy. Linguistic validation of the DN4 for use in international studies has been reported as well [32]. We concluded that the validated Turkish version of the DN4 is a reliable, valid, and fast screening test for detecting neuropathic pain. The DN4 takes only 1 minute to perform and has a sensitivity of 95% and a specificity of 96.6% [15]. Painful diabetic neuropathy is also diagnosed using the DN4 test [33]. We used OAB-V8 to diagnose OAB, which includes the first 8 questions of the OAB-questionnaire and proposed OAB screening. Acquadro et al. [34] translated it into 14 languages in 2006 and linguistic validations were completed. The OAB-V8 is a short, efficient, and usable screening tool, and the validity of its Turkish version has been demonstrated [16].
In conclusion, we found that there is significant correlation between OAB and diabetic peripheral neuropathy among DM patients by using noninvasive, short, and easily applicable questionnaires. This finding helps improve the understanding of the characteristics of representative DM complications. This correlation might give clinicians a good and early opportunity to evaluate the risk of other DM complications using simple and easy methodologies.
Notes
Research Ethics
This work was approved by the Ethics Committee of Bozok University, Medicine Faculty (13.03.2015/79), and all patients provided written informed consent.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.