Voluntary Wheel Running Improves Spatial Learning Memory by Suppressing Inflammation and Apoptosis via Inactivation of Nuclear Factor Kappa B in Brain Inflammation Rats
Article information
Abstract
Purpose
Exercise has been shown to protect against diverse brain diseases. Voluntary exercise improves cognition and has a neuroprotective effect. The aim of this investigation is to study the effect of voluntary wheel running on brain inflammation in rats with regard to inflammation and apoptosis.
Methods
Brain inflammation was caused by intracranial injection of lipopolysaccharide using a stereotaxic instrument. Voluntary wheel running group were conducted during 21 consecutive days, staring 2 days after brain inflammation.
Results
Brain inflammation increased proinflammatory cytokine production and apoptosis cell death in the hippocampus. There changes in the hippocampus deteriorated spatial learning memory. However, voluntary wheel running suppressed the secretion of inflammatory cytokines and apoptotic neuronal cell death via inactivation of nuclear factor kappa B (NF-κB)/NF-κB inhibitor-α pathway. Voluntary wheel running also promoted the recovery of the spatial learning memory impairment.
Conclusions
Voluntary wheel running after brain inflammation enhanced spatial learning memory by suppressing proinflammatory cytokine secretion and apoptosis cell death. Voluntary wheel running is also expected to be effective in inflammatory diseases of the urogenital system.
• HIGHLIGHTS
- Administration of LPS caused brain inflammation and showed spatial learning memory impairment.
- Administration of LPS increased secretion of proinflammatory cytokines and apoptotic neuronal cell death.
- Voluntary wheel running suppresses inflammation and apoptosis, resulting in improvement of spatial learning memory in brain inflammation rats.
INTRODUCTION
Neuroinflammation acts as a cause of various neurodegenerative diseases and adversely affects cognitive function and neuroplasticity [1,2]. Single cerebrospinal injection of a bacterial endotoxin called lipopolysaccharide (LPS) in rodents leads to neuro-inflammatory animal model that causes cognitive dysfunction and learning deficits [3]. LPS is an endotoxin in the cell wall of gram-negative bacteria and has been used to evoke inflammation in the nervous system.
LPS releases tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), which causes inflammation-mediated neuronal damage [4]. Moreover, neuroinflammation induced by LPS showed excessive apoptosis in the brain [5,6]. LPS enhances proinflammatory cytokine secretion by stimulating nuclear factor kappa B (NF-κB) [7,8]. NF-κB serves as a basis for apoptosis cell death in neuroinflammation [9,10]. Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining for finding of DNA fragmentation, caspase-3 expression acting as an executor of apoptosis, and imbalance of B-cell lymphoma 2 (Bcl-2)-associated X protein (Bax) versus Bcl-2 are biological markers representing apoptosis [6,11]. LPS-induced brain inflammation increased TUNEL-positive and caspase-3-positive cell number, improved Bax expression, and attenuated Bcl-2 expression, thereby enhancing the Bax versus Bcl-2 ratio in the motor cortex [6].
Exercise is known to reduce neurological impairment, promote learning ability and memory capability, and inhibit apoptotic neuronal cell death [11]. Voluntary exercise improves cognitive ability, promotes antiapoptotic action, and has neuroprotective effect [12]. Compared to the treadmill exercise, which is a forced exercise, voluntary wheel running is known to be safe from systemic stress and does not reduce the neuroprotective effect [12,13]. Therefore, in this study, we evaluated spatial learning memory following NF-κB activation in LPS-induced brain inflammation. We also observed the effect of voluntary wheel running on NF-κB expression, inflammation, apoptotic cell death, and spatial learning memory impairment.
MATERIALS AND METHODS
Animals
For this experiment, 8-week-old male Sprague-Dawley rats (220±10 g, n=30) were bought from Orient Bio Co. (Seongnam, Korea). The animals were divides into three groups (n=10 in each group): sham-operation group, brain inflammation-induction group, and brain inflammation-induction and voluntary wheel running group. This experimental procedure received approval number from the Institutional Animal Care and Use Committee of Kyung Hee University (KHUASP[SE]-17-095).
Brain Inflammation
After anesthetizing by Zoletil 50 (10 mg/kg intraperitoneally; Virbac Laboratories, Carros, France), the rats were put on a stereotaxic frame. As explained next [14], a 26-G needle from Hamilton syringe (Micro 701; Hamilton Co., Reno, NV, USA) was inserted into the cerebral ventricle through a hole drilled in the skull. LPS (055:B5; Sigma Aldrich, St Louis, MO, USA) 50 μg was dissolved in 7-μL physiological saline and injected into the cerebral ventricle for at least 3 minutes. The rats in the sham-operation group received same amount of physiological saline in the same way, except for LPS.
Wheel Running Protocol
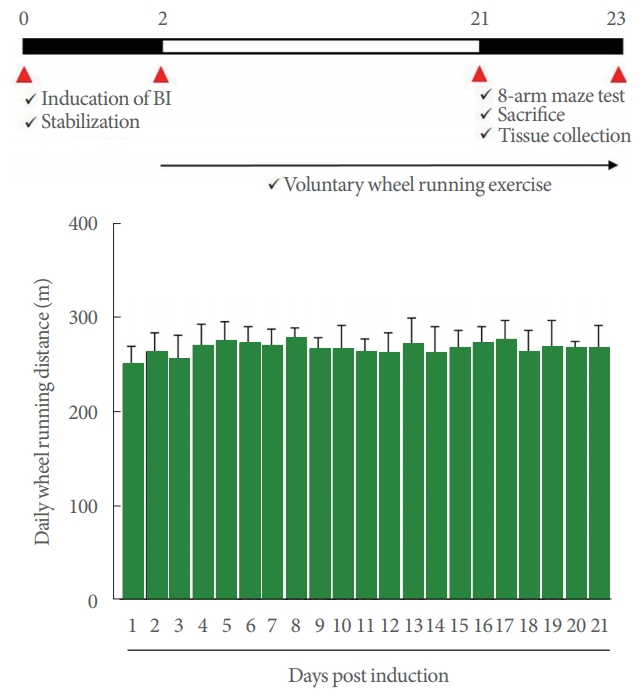
The rats in the voluntary wheel running group started exercising 2 days after causing brain inflammation and continued exercising for 3 weeks. As explained next [6], the rats in the voluntary wheel running group were separated and placed alone in the cages with running wheels (20 in diameter ×9 cm in width). The number of wheel revolution was recorded by an electronic sensor attached to the running wheel, and the mileage was calculated (Fig. 1). The wheels were only allowed to move for 12 hours (08:00 AM to 20:00 PM) to prevent the excessive running of the experimental animals.
Radial 8-Arm Maze Test
Spatial learning memory was measured by a radial 8-arm maze test, as explained next [15], and the device for a radial 8-arm maze has a central diameter of 30 cm, a length of 50 cm, and a width of 10 cm. A small container (3-cm diameter ×1-cm depth) filled with 100 μL of water was put at the end of the arm to attract the animals to each arm. The rats were not watered for 24 hours thereafter, and the rats lacking water were allowed to find water in the maze. At the 23 days after brain surgery to induce brain inflammation, the test was performed. The evaluation was analyzed by the time spent finding water in all 8-arm mazes and the error of re-entering the visited arm. Furthermore, the correct number of choices was calculated before the first error. The test ended after 8 minutes had elapsed.
Tissue Preparation
After anesthesia by Zoletil 50 (10 mg/kg; Vibac Laboratories, Carros, France), the rats were infused with 50mM phosphatebuffered saline through heart and were treated by 4% paraformaldehyde for fixation. After removing the brains, 40-μm-thick coronal sections were prepared with a frozen microtome (Leica, Nussloch, Germany).
Proinflammatory Cytokines
Enzyme-linked immunoassay was done to detect the concentrations of TNF-α and IL-6 in the hippocampus. The procedure was performed using an enzyme immunoassay kit (Abcam, Cambridge, UK) following the manufacturer’s protocol.
Western Blotting
Western blot was done as explained next [16,17]. The hippocampal tissue was homogenized on chilled lysis buffer (Cell Signaling Technology, Inc., Danvers, MA, USA) with 1 mM phenylmethylsulfonyl fluoride (Sigma Aldrich), and then centrifuged at 14,000 rpm for 30 minutes at 4°C. For the primary antibodies, anti-rabbit antibody for NF-κB-α (1:1,000; Abcam), NF-κB inhibitor-α (IκB-α) (1:1,000; Santa Cruz Biotechnology, CA, USA), anti-mouse antibody for Bax, Bcl-2, and β-actin (1:1,000; Santa Cruz Biotechnology) were included. Horseradish peroxidase-conjugated anti-rabbit antibody for NF-κB-α, IκB-α, and horseradish peroxidase-conjugated anti-mouse antibody for Bax, Bcl-2, and β-actin were included as the secondary antibodies. To compare the relative protein expression, the bands were calculated densitometrically by Image-Pro plus computer-assisted image analysis system (Media Cyberbetics Inc., Silver Spring, MD, USA).
TUNEL Assay
TUNEL assay was done following to the manufacturer’s protocol using In Situ Cell Death Detection Kit (Roche, Mannheim, Germany) as explained next [17]. TUNEL-positive cells were visualized by treating with 0.05% 3,3-diaminobenzidine with 0.01% H2O2 in 50 mM Tris-buffer (pH, 7.6) for 10 minutes. For the counter-staining, Nissls (DAKO, Glostrup, Denmark) was used.
Cleaved Caspase-3 Immunohistochemistry
Immunohistochemistry for cleaved caspase-3 was done as explained next [6,17]. The sections were treated with rabbit anticleaved caspase-3 antibody (1:500; Cell Signaling Technology, Inc.) during overnight, and then incubated with biotinylated rabbit secondary antibody (1:200; Vector Laboratories) for another 1 hour. After amplification of the secondary antibody with the Vector Elite ABC kit (1:100; Vector Laboratories), 0.03% diaminobenzidine was used to visualized the antibodybiotin-avidin-peroxidase complex.
Statistical Analysis
The number of TUNEL-positive and cleaved caspase-3-positive cells was presented as the number of cells per mm2 in the hippocampal dentate gyrus. The data were analyzed with 1-way analysis of variance with Duncan post hoc test using IBM SPSS Statistics ver. 23.0 (IBM Co., Armonk, NY, USA).
RESULTS
Spatial Learning Memory
The results of the spatial learning memory are presented in Fig. 2. Intracranial ventricular injection of LPS showed longer completed time, more error number, and less correct number (P<0.05). In contrast, voluntary wheel running showed shortened completed time, reduced error number, more correct number in the rats with brain inflammation-induction (P<0.05).
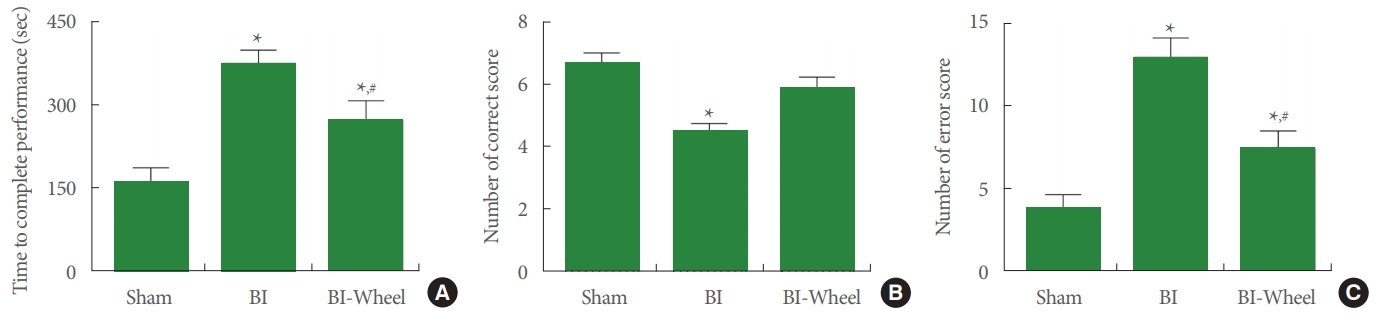
Performance time, correct number, error number in the radial 8-arm maze test. (A) Time to complete performance. (B) Correct score. (C) Error score. Sham, sham-operation group; BI, brain inflammation-induction group; BI-Wheel, brain inflammation-induction and voluntary wheel running group. *P<0.05 when compared to the sham-operation group. #P<0.05 when compared to the brain inflammation-induction group.
Proinflammatory Cytokines
The results of the expression levels of TNF-α and IL-6 in the hippocampus are presented in Fig. 3. Intracranial ventricular injection of LPS enhanced TNF-α and IL-6 expressions (P < 0.05). In contrast, voluntary wheel running inhibited the expression of TNF-α and IL-6 in the rats with brain inflammation-induction (P<0.05).
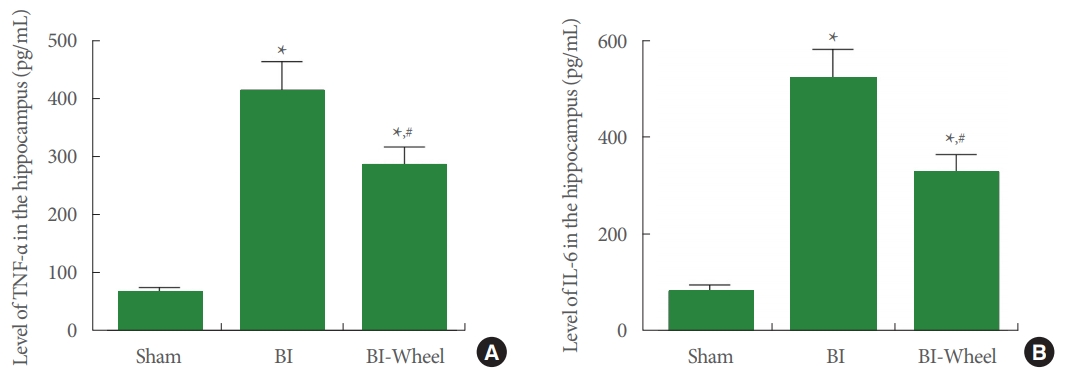
Concentrations of tumor necrosis factor (TNF)-α and interleukin (IL)-6 in the hippocampus. (A) Expression of TNF-α. (B) Expression of IL-6. Sham, sham-operation group; BI, brain inflammation-induction group; BI-Wheel, brain inflammation-induction and voluntary wheel running group. *P<0.05 when compared to the sham-operation group. #P<0.05 when compared to the brain inflammation-induction group.
NF-κB and IκB-α
The results of the Western blotting determining NF-κB activation and IκB-α phosphorylation in the hippocampus are presented in Fig. 4. Intracranial ventricular injection of LPS increased IκB-α phosphorylation and NF-κB expression (P < 0.05). In contrast, voluntary wheel running decreased IκB-α phosphorylation as well as NF-κB in expression in the rats with brain inflammation-induction (P<0.05).
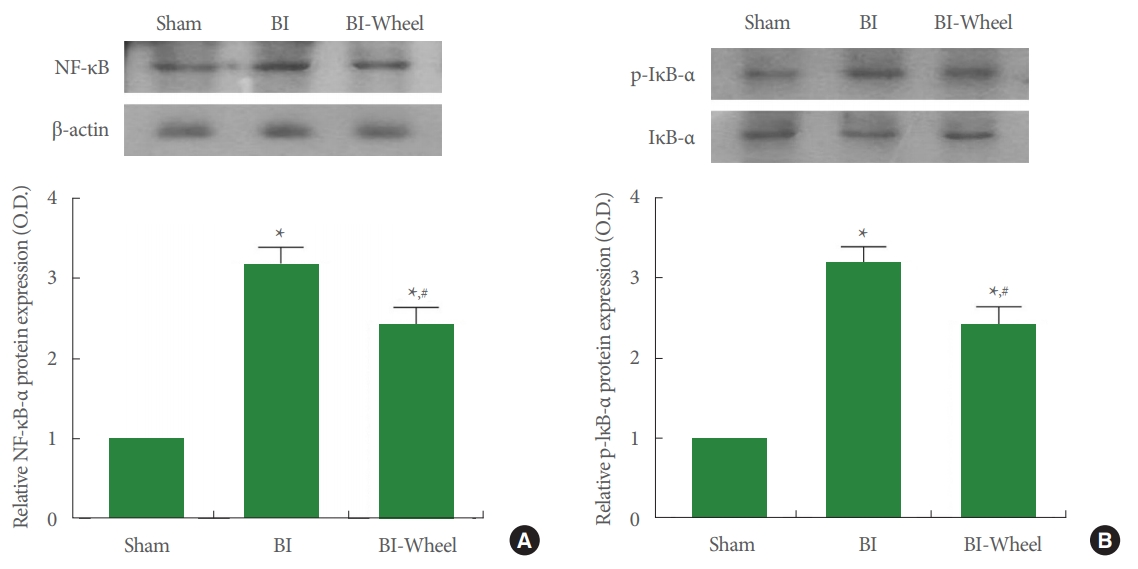
Expression of nuclear factor-κB (NF-κB) and NF-κB inhibitor-α (IκB-α) in the hippocampus. Actin was used as an internal control (46 kDa). (A) Expression of NF-κB. Upper panel: representative expression of NF-κB. Lower panel: relative expression of NF-κB. (B) Expression of IκB-α. Upper panel: representative expression of IκB-α. Lower panel: relative expression of IκB-α. Sham, sham-operation group; BI, brain inflammation-induction group; BI-Wheel, brain inflammation-induction and voluntary wheel running group. *P<0.05 when compared to the sham-operation group. #P<0.05 when compared to the brain inflammation-induction group.
Apoptotic Neuronal Cell Death
The results of TUNEL staining and cleaved caspase-3 expression in the hippocampal dentate gyrus are presented in Fig. 5A. Bax and Bcl-2 expression in the hippocampus are presented in Fig. 5B. Intracranial ventricular injection of LPS increased the number of TUENL-positive and cleaved caspase-3 cells and ratio of Bax/Bcl-2 (P<0.05). In contrast, voluntary wheel running suppressed the number of TUENL-positive and cleaved caspase-3-positive cells and the ratio of Bax/Bcl-2 in the rats with brain inflammation-induction.
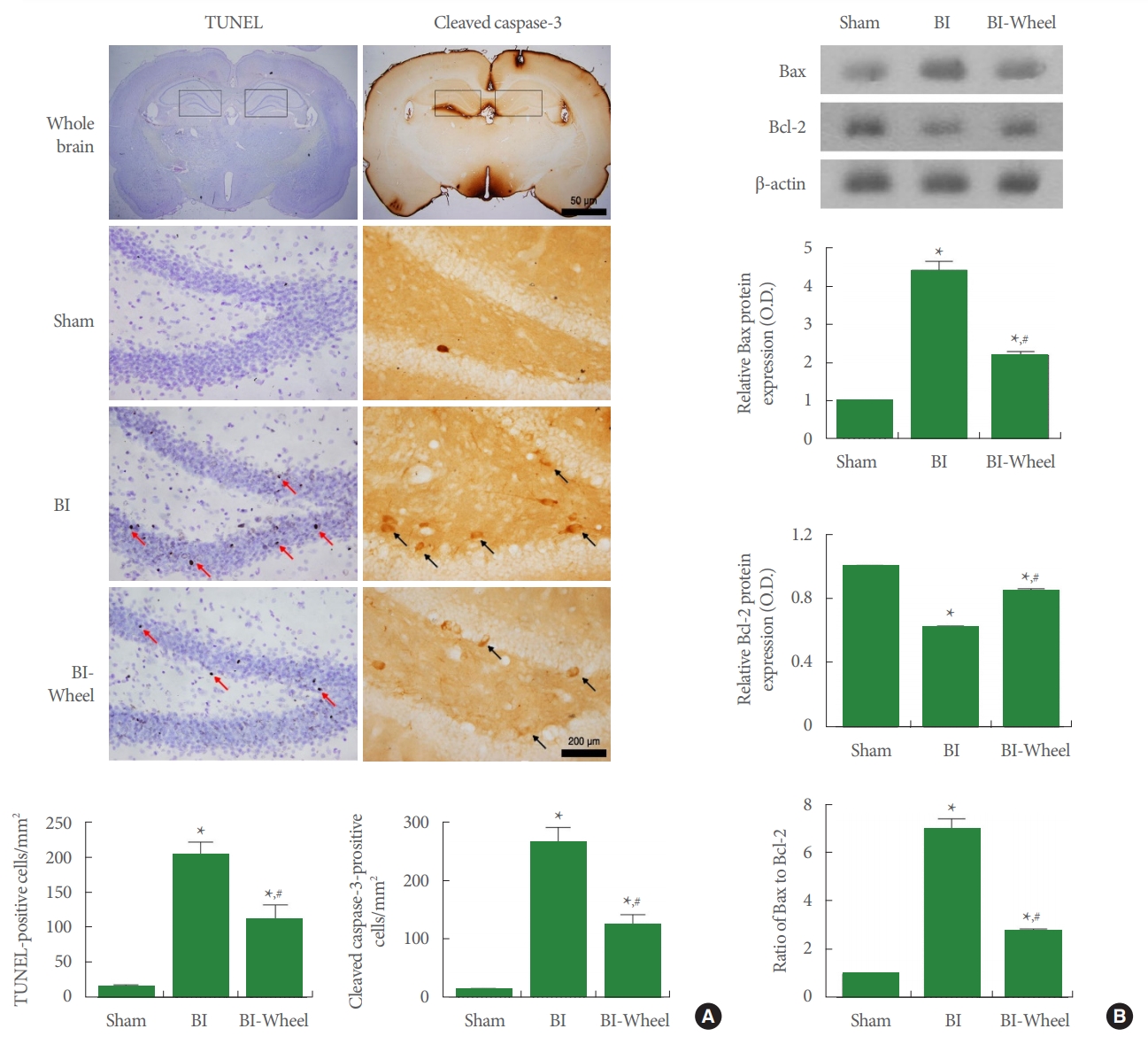
Apoptotic cell death in the hippocampus. (A) Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL)-positive cells and cleaved caspase-3-positive cells. Upper panel: photomicrographs of TUNEL-positive cells and cleaved caspase-3-positive cells. □ shows counting area in the hippocampus. Red arrows indicate TUENL-positive cells. Black arrows indicate cleaved caspase-3-positive cells. Lower panel: the number of TUNEL-positive cells and cleaved caspase-3-positive cells. (B) B-cell lymphoma 2 (Bcl-2)-associated X protein (Bax) and Bcl-2 expression in the hippocampus. Upper panel: representative expression of Bax and Bcl-2. Actin was used as an internal control (46 kDa). Lower panel: relative expression of Bax and Bcl-2. Sham, sham-operation group; BI, brain inflammation-induction group; BI-Wheel, brain inflammation-induction and voluntary wheel running group. *P<0.05 when compared to the sham-operation group. #P<0.05 when compared to the brain inflammation-induction group.
DISCUSSION
Inflammation and apoptosis serve as the underlying mechanisms of brain inflammation, leading to cognitive impairment and memory deficits [2,3,14]. In the current study, brain inflammation worsened spatial learning memory. However, voluntary wheel running spatial learning memory impairment caused by brain inflammation.
NF-κB regulates immunity and inflammation in diseases such as neuroinflammation [18]. NF-κB is activated by IκB-α degradation by signaling-induction, and activation of NF-κB pathway regulates the transcription of inflammatory genes, including proinflammatory cytokine expression in brain inflammation [10]. In the current study, neuroinflammation caused by LPS enhanced IκB-α phosphorylation, resulting in NF-κB activation. Enhancement of NF-κB eventually led to increased expression of TNF-α and IL-6 in hippocampus. The current results indicated that overexpression of proinflammatory cytokine aggravated the progression of brain inflammation.
Physical exercise to maintain and improve nerve health can be a very effective treatment strategy for preventing neuroinflammation-related diseases [19]. Lymphocytes increase during and after exercise, and exercise regulates the balance of secretion of proinflammatory and anti-inflammatory cytokines [20,21]. It is well known that physical exercise inhibits the activation of NF-κB signal caused by inflammation [22,23]. In particular, voluntary wheel exercise in rodents induces changes in several signaling molecules by inhibiting the stress-related behavior compared to forced exercise [24,25]. In the current study, voluntary wheel running reduced IκB-α phosphorylation, resulting in NF-κB inactivation. Inactivation of NF-κB suppresses the production of proinflammatory cytokines such as TNF-α and IL-6 in the hippocampus.
LPS-induced inflammatory response can trigger signaling pathways of apoptosis in the brain [2,26,27]. After brain inflammation, the imbalance of Bcl-2 and Bax increases the release of mitochondrial intermembrane proteins, including cytochrome c, which activates caspase-3 and DNA fragmentation [12,28]. In particular, stimulation of the NF-κB pathway by cellular stress promotes apoptosis-induced cell death [29]. Moreover, several proinflammatory cytokines can directly induce apoptotic cell death through specialized signaling pathways [26]. In the current study, intracranial ventricular injection of LPS enhanced the number of TUNEL-positive cells, the expression of cleaved caspase-3, and the ratio of Bax/Bcl-2, demonstration that brain inflammation aggravated the apoptotic process.
Many methods have been implemented to treat neuroinflammation, among which physical exercise is recommended as a treatment for brain inflammation [14,19,30]. In particular, voluntary exercise down-regulates the expression of glutamate receptors associated with excitotoxicity [31]. In the current study, voluntary wheel running suppressed the brain inflammationinduced increased DNA fragmentation and cleaved caspase-3 expression in the hippocampal dentate gyrus. Voluntary wheel running reduced Bax expression and increased Bcl-2 expression in the hippocampus. Voluntary wheel running also inhibited the expression of NF-κB and proinflammatory cytokines through inhibiting IκB-α phosphorylation.
Here in the current study, we found that voluntary wheel running after brain inflammation enhanced spatial learning memory by inhibiting the secretion of proinflammatory cytokines and cell death by apoptosis in the hippocampus. Voluntary wheel running was shown to be effective by reducing brain inflammation-induced activation of NF-κB/IκB-α pathway. Voluntary wheel running is also expected to be effective in inflammatory diseases of the urogenital system.
Notes
Research Ethics
This experimental procedure was approved by the Institutional Animal Care and Use Committee of Kyung Hee University (KHUASP[SE]-17-095).
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTION STATEMENT
·Conceptualization: IGK
·Data curation: JYK
·Formal Analysis: JYK
·Funding acquisition: IGK
·Methodology: JYK
·Project Administration: IGK
·Visualization: IGK
·Writing – Original Draft: IGK
·Writing – Review & Editing: IGK