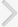|
 |


- Search
| Int Neurourol J > Volume 20(3); 2016 > Article |
|
ABSTRACT
Purpose
To report the prevalence and risk factors of stress urinary incontinence (SUI) and the prevalence of intrinsic sphincter deficiency in women with multiple sclerosis (MS).
Methods
We conducted a retrospective study. Female patients with MS, followed for lower urinary tract symptoms (LUTS) during a 15-year period were included. Demographic data, MS history, expanded disability status scale (EDSS) score at the urodynamic visit, obstetrical past, birth weight, LUTS, and urodynamic findings were collected. SUI was defined as incontinence during cough, or any effort. A maximum urethral closure pressure less than 30 cm H2O defined intrinsic sphincter deficiency.
Results
We included 363 women with a mean age of 46.7±10.8 years and a mean disease duration of 12.9±8.7 years. The incidence of relapsing remitting MS, a secondary progressive form, and a primary progressive form was 60.6%, 32.8%, and 6.6%, respectively. The prevalence of SUI was 31.4%. The prevalence of intrinsic sphincter deficiency was 1.4% and 0.8% of these patients had a SUI (P=0.300). In a multivariate analysis, women with a SUI had significantly higher birth weight (P=0.030), a pelvic organ prolapse (P=0.021), urgent urinary incontinence (P=0.006), a lower EDSS score (P=0.019), and a weaker containing effort (P<0.001).
Multiple sclerosis (MS) is an autoimmune, inflammatory, chronic disease that results in demyelinating lesions of the central nervous system. It affects women 3 times more often than men [1]. About 50% to 90% of patients suffer from lower urinary tract symptoms (LUTS) during the course of the disease [2] and 90% of the patients have LUTS after 10 years of the disease [3,4]. Urgency and urge incontinence are most frequently reported [2]. Urinary dysfunction may affect social relationships and activities of these patients. Moreover, urinary disorders can sometimes lead to permanent urological alterations [2].
Stress urinary incontinence (SUI), defined as the involuntary leakage of urine on effort or exertion by the International Continence Society, is common in the general adult female population [5]. The prevalence ranges from 17% to 41% [6]. Two mechanisms can explain the SUI: bladder neck hypermobility and intrinsic urethral sphincter deficiency. Many risk factors of SUI like pregnancy, obesity, pelvic organ prolapse (POP), and obstetrical surgeries are known in the general population [6]. SUI has severe implications for daily function, social interactions, and psychological wellbeing [6].
Few studies have reported the prevalence of SUI in MS and this has varied from 16% to 55.9% [7-9]. These studies have investigated some factors relating to SUI in MS: female patients with SUI were older, had more of a relapsing remitting MS (RRMS), a higher voided volume and average maximum urinary flow, and a higher body mass index than MS patients without SUI. Only one study evaluated expanded disability status scale (EDSS) score [8], and only one reported MS relating to SUI [7]. To our knowledge, the prevalence of intrinsic urethral sphincter deficiency has not been studied previously in these patients.
Thus, the present analysis was undertaken to determine the prevalence of SUI, the prevalence of intrinsic urethral sphincter deficiency, and risk factors of SUI in women with MS.
We conducted a retrospective, single site study, at a large dedicated rehabilitation MS center in Saint Philibert Hospital in Lille, after receiving local Institutional Review Board approval. We reviewed the urodynamic database of women with MS over 15 years from December 1999 to June 2014. All women aged >18 years with MS, who had undergone a urodynamic test for LUTS were included. We collected demographic information, physical examination findings, and urodynamic data. Demographic information included MS classification (RRMS, secondary progressive MS [SPMS] and primary progressive MS [PPMS]), age and EDSS score at the visit, age of onset of MS, obstetrical history (uterine status, parity, type of delivery, birth weight, episiotomy, and forceps), number of urinary tract infections per year, constipation, number of sanitary pads per day, and if patients had pelvic floor muscle training. LUTS like SUI, urgency, urge incontinence, frequency of voiding, voiding dysfunction, and urinary retention were recorded. SUI was defined by the International Continence Society as the involuntary leakage of urine on effort, exacerbation, sneezing, or coughing [5]. Pelvic examination findings were recorded. Prolapse was defined using the POP quantification system [10]. A rehabilitation physician, who had urodynamic experience with a standard multichannel urodynamic setup using the the Medtronic system (Minneapolis, MN, USA), performed urodynamics. Urodynamic methodology described by Amarenco and De Sèze for neurogenic bladder was used [2,11].
Urodynamic information included a uroflowmetry (flow rate, residual urine volume by catheterization), a cystometry (bladder compliance, intravesical pressure, sensation of bladder filling, and cystometric capacity), and profilometry (maximum urethral closure pressure, urethral pressure during a straining effort). The intrinsic urethral sphincter deficiency was defined by a maximum urethral closure pressure <30 cm H2O [12]. If the patient had performed several urodynamic tests, only the last one was recorded.
All data analysis was made using the R software (R Foundation for Statistical Computing, Vienna, Austria) [13]. First, we performed a descriptive analysis with means, medians, interquartile ranges for continuous variables, and frequencies for noncontinuous measures. Then, patients with SUI were compared to patients without SUI for the different variables. Univariate analysis was performed using Student t-test or MannWhitney-Wilcoxon for quantitative variables and the chi-square test or Fisher exact test for qualitative data. Variables were included in the logistic regression model examining factors associated with SUI in MS. Conditional forward and backward models were performed on Akaike Information Criterion. These criteria estimate the quality of each model, relative to each of the other models. The model with the lowest Akaike Information Criterion value indicates a higher quality. Receiver operating characteristic curves of the forward and backward models were performed. Significance level was set at P<0.05.
During this period, 400 women with MS were referred to our center. Thirty-five women with an incomplete file and 2 patients with an indwelling catheter were excluded. The mean age of 363 patients was 46.7±10.8 years and the mean disease duration was 12.9±8.7 years. Median patient disability expressed as an EDSS score was 5 (3.5). The incidence of patients with a RRMS, SPMS, and PPMS was 60.6%, 32.8%, and 6.6%, respectively.
The prevalence rate of SUI in our study was 31.4% in patients with MS. The mean age of patients with SUI was 46.4±11.1 years and 46.8±10.7 years for patients without SUI (P=0.722). Table 1 summarizes the distribution of prevalence of SUI by age range. Clinical characteristics of patients with or without SUI are shown in Table 2.
Women with RRMS were younger than women with PPMS or SPMS (P<0.001), and had a lower EDSS score (P<0.001) (Table 3).
Patients with PPMS were more likely to have recent onset disease (P<0.001) as compared to patients with SPMS and RRMS.
Women with SUI had more pregnancies (P<0.002) and childbirths (P<0.003) than patients without SUI. In parous women, SUI significantly increased with episiotomy (P<0.001) and birth weight (P=0.037) if birth weight was >4 kg, if birth weight was between 3 to 4 kg (P=0.031). Patients with no SUI presented with significantly less delivery disorders than women with SUI (P<0.001). Patients with SUI had more pelvic floor muscle training than patients with no SUI (P=0.026).
Table 4 summarizes LUTS. For pelvic prolapse, 4 patients had an anterior prolapse, 3 had a posterior and anterior prolapse, and 1 had an anterior middle, and posterior prolapse.
In our study, urodynamic findings showed several significant differences between women with and without SUI. These results are shown in Table 5. The prevalence of intrinsic urethral sphincter deficiency was 1.4% of MS patients, and there was no association with SUI.
Conditional forward stepwise model was compared to conditional backward stepwise model. The results of multivariate analysis of backward model are shown in Table 6. The 2 models showed one different variable, which was not significantly associated with SUI (number of pregnancies for forward model and episiotomy for backward model). Fig. 1 showed receiver operating characteristic curves of the 2 models. Backward model had a better sensitivity and lower specificity than the forward model.
LUTS are very common in MS and over 90% of patients present with these symptoms, 10 years from disease onset [2]. The prevalence of SUI in our study was 31.4%. The mean age and mean disease duration are the same in other series of urological disorders with MS [4,14]. Our department is a large and specialized center for follow-up of MS patients in the North of France. In the MS population, urodynamic investigations are necessary to better understand LUTS and choose the best treatment. Rehabilitation physicians at our center, had urodynamic experience with MS patients. To our knowledge, this is the first study reporting on the prevalence rate of SUI in a large dedicated MS rehabilitation center.
Three studies determined the prevalence of SUI in female patients with MS, which ranged from 16% to 55.9% [7-9]. These 3 studies reported the prevalence of SUI in MS in a urologic care center, but only 43% of MS patients were under the care of a urologist. Moreover, MS populations in those studies were older than in our analysis. Furthermore, SUI was diagnosed with a questionnaire, but sometimes, patients with MS cannot write due to motor or sensory deficiencies. Only one study recorded the EDSS score and another MS subtype [7,8]. These limitations may have contributed to a selection bias.
SUI is the most common subtype of incontinence in the general female population and the prevalence rate is between 17%–41% [6]. SUI increases with age in the general population, but in our study, SUI affects young women too. Some risk factors of SUI in MS are common in the general population like a birth weight >4 kg, a POP, and the number of pregnancies [15-17]’s. The risk of SUI was increased 8 fold by a birth weight >4 kg and by a POP. For POP, Dillon et al. [7]’s did not report a significant difference between MS patients with or without SUI. The prevalence of POP in our study was smaller than in the general population, and Dillon et al.’s population. The type of care centers, and the older population in Dillon et al. [7]’s study may explain these differences. In the general population, like in our study, anterior prolapses seem to be more common [17]. The number of pregnancies was only associated with SUI in a univariate analysis. Dillon et al. [7] did not report the effect of pregnancy on SUI in the MS population. Futhermore, Durufle et al. [18]. showed in his retrospective study that delivery modalities had no influence on the frequency of urinary disorders, or the type of problems in MS.
We reported specific risk factors of SUI due to MS. It was the first study reporting prevalence of SUI in MS with EDSS score, clinical form of MS, treatments, and disease duration. A smaller EDSS score, RRMS, urge incontinence, a bigger volume of strong desire to void, and women who had taken anticholinergic treatment were significantly associated with SUI.
In Dillon et al. [7]’s study, RR form of MS was associated with SUI. Patients in our population, with RRMS were younger and had a lower EDSS score. Women with a RRMS would tend to be more active than other women with a progressive form of MS. Furthermore, progressive forms tend to decrease the neurological function more rapidly. Ukkonen et al. [19] showed that detrusor sphincter dyssynergia is possibly more frequent in the progressive form, making patients less likely to have SUI clinically.
Urge incontinence was significantly associated with SUI in our study. This symptom is frequent in MS and Guinet-Lacoste et al. [8] have showed an association between SUI and detrusor hyperactivity with the Urinary Symptom Profile questionnaire. Patients with SUI take anticholinergics frequently. The frequent association between urge incontinence and SUI may explain the result. Furthermore, anticholinergic medication is frequently prescribed for hyperactivity symptoms like urgency or urge incontinence. The administration of empirical anticholinergic treatment could lead to a SUI because it increases maximal vesical capacity and decreases frequency of voiding.
It is the first time that a containing effort was studied in the MS population. A weak containing effort was associated with SUI. Dompeyre et al. [20] showed that women with SUI in the general population did not have a more efficient increase of urethral closure pressure in a containing effort, than women without SUI. Furthermore, fatigue is a common symptom of MS and may interfere with the function of the pelvic floor muscles [21].
The prevalence of intrinsic urethral sphincter deficiency was 1.4% in our population, and it was not associated with SUI. This finding suggests that urethral sphincter is intact in these patients, but SUI may be due to a hypermobility of the bladder neck. Muscular contractions of pelvic floor muscles may be less efficient and fatigue may decrease the strength and time of contractions, and sensory deficiencies could affect realization of contractions.
SUI had a significant impact on the lives of patients with MS. Women with SUI indicated that this symptom had a moderate or great impact on their physical activity. Specifically looking out for SUI in the medical examination could improve the treatment in this population. Pelvic floor muscle training seems to be effective for urinary disorders in MS [22] and especially for decreasing urinary incontinence. Surgical treatment of MS patients with SUI has never been evaluated. Voiding dysfunction is a postsurgical risk in the general population. The European Association of Urology guidelines recommend that patients, who have a neurogenic SUI should be able to perform self-catheterization before surgery [23].
There are several limitations of this study. We confirmed SUI only by medical history and not by urodynamic testing.
Furthermore, there was no measure of physical activity and no assessment of ethnicity in our study, which are common risk factors in the general population. Few variables like childhood enuresis, arterial hypertension, and obesity could not be tested because the number of patients was insufficient. Finally, the study design was subject to a degree of selection bias, since we only included women coming to our center.
Prevalence rate of SUI in female patients with MS was 31.4%. A targeted screening seems to be essential to diagnose SUI in these patients. Younger, active, mothers with RRMS have a greater risk of suffering from SUI. Intrinsic sphincter deficiency does not seem to be the cause of SUI in MS. Pelvic floor muscle training is probably an effective treatment for this symptom.
NOTES
REFERENCES
1. Kingwell E, Marriott JJ, Jetté N, Pringsheim T, Makhani N, Morrow SA, et al. Incidence and prevalence of multiple sclerosis in Europe: a systematic review. BMC Neurol 2013;13:128. PMID: 24070256




2. Amarenco G, de Sèze M, Ruffion A, Sheikh Ismael S. Clinical and urodynamic evaluations of urinary disorders in multiple sclerosis. Ann Phys Rehabil Med 2014;57:277-87. PMID: 24980885


3. De Seze M, Game X. Multiple sclerosis and pelviperineology: urinary and sexual dysfunctions and pregnancy. Prog Urol 2014;24:483-94. PMID: 24875567


4. Betts CD, D’Mellow MT, Fowler CJ. Urinary symptoms and the neurological features of bladder dysfunction in multiple sclerosis. J Neurol Neurosurg Psychiatry 1993;56:245-50. PMID: 8459239



5. Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology 2003;61:37-49. PMID: 12559262


6. Gasquet I, Tcherny-Lessenot S, Gaudebout P, Bosio Le Goux B, Klein P, Haab F. Influence of the severity of stress urinary incontinence on quality of life, health care seeking, and treatment: a national cross-sectional survey. Eur Urol 2006;50:818-25. PMID: 16678340


7. Dillon BE, Seideman CA, Lee D, Greenberg B, Frohman EM, Lemack GE. A surprisingly low prevalence of demonstrable stress urinary incontinence and pelvic organ prolapse in women with multiple sclerosis followed at a tertiary neurogenic bladder clinic. J Urol 2013;189:976-9. PMID: 23022010


8. Guinet-Lacoste A, Verollet D, Le Breton F, Peyrat L, Amarenco G. Incontinence urinaire à l’effort et sclérose en plaques. Ann Phys Rehabil Med 2014;57:e308-9. 
9. Murphy AM, Bethoux F, Stough D, Goldman HB. Prevalence of stress urinary incontinence in women with multiple sclerosis. Int Neurourol J 2012;16:86-90. PMID: 22816049



10. Bader G, Koskas M. Prolapsus des organes pelviens. Du symptôme à la prise en charge thérapeutique. EMC - AKOS (Traité Méd) 2008;3:1-8. 
11. Amarenco G, Kerdraon J, Denys P. Bladder and sphincter disorders in multiple sclerosis. Clinical, urodynamic and neurophysiological study of 225 cases. Rev Neurol (Paris) 1995;151:722-30. PMID: 8787103

12. Hermieu JF; Comite d’Urologie et de Pelvi-perinéologie de la Femme Association Franņaise d’Urologie. Recommendations for the urodynamic examination in the investigation of non-neurological female urinary incontinence. Prog Urol 2007;17(6 Suppl 2):1264-84. PMID: 18214138

13. The R Project for Statistical Computing [Internet]; The R Foundation; 2013 [cited 2015 Aug 1]. Available from: http://www.R-project.org/.
14. Giannantoni A, Scivoletto G, Di Stasi SM, Grasso MG, Vespasiani G, Castellano V. Urological dysfunctions and upper urinary tract involvement in multiple sclerosis patients. Neurourol Urodyn 1998;17:89-98. PMID: 9514141


15. Rortveit G, Daltveit AK, Hannestad YS, Hunskaar S; Norwegian EPINCONT Study. Urinary incontinence after vaginal delivery or cesarean section. N Engl J Med 2003;348:900-7. PMID: 12621134


16. Rortveit G, Hannestad YS, Daltveit AK, Hunskaar S. Age- and type-dependent effects of parity on urinary incontinence: the Norwegian EPINCONT study. Obstet Gynecol 2001;98:1004-10. PMID: 11755545


17. Adjoussou SA, Bohoussou E, Bastide S, Letouzey V, Fatton B, de Tayrac R. Functional symptoms prevalence and anatomo-functional associations on women with genital prolapse. Prog Urol 2014;24:511-7. PMID: 24875570


18. Durufle A, Nicolas B, Petrilli S, Robineau S, Guille F, Edan G, et al. Effects of pregnancy and childbirth on the incidence of urinary disorders in multiple sclerosis. Clin Exp Obstet Gynecol 2006;33:215-8. PMID: 17211968

19. Ukkonen M, Elovaara I, Dastidar P, Tammela TL. Urodynamic findings in primary progressive multiple sclerosis are associated with increased volumes of plaques and atrophy in the central nervous system. Acta Neurol Scand 2004;109:100-5. PMID: 14705971


20. Dompeyre P, Fritel X, Fauconnier A, Robain G. Pelvic floor muscle contraction and maximum urethral closure pressure. Prog Urol 2015;25:200-5. PMID: 25468000


21. Andreasen AK, Jakobsen J, Petersen T, Andersen H. Fatigued patients with multiple sclerosis have impaired central muscle activation. Mult Scler 2009;15:818-27. PMID: 19465444


22. Gaspard L, Tombal B, Castille Y, Opsomer RJ, Detrembleur C. Pelvic floor muscles training, electrical stimulation, bladder training and lifestyle interventions to manage lower urinary tract dysfunction in multiple sclerosis: a systematic review. Prog Urol 2014;24:222-8. PMID: 24560290


23. Lucas MG, Bosch RJ, Burkhard FC, Cruz F, Madden TB, Nambiar AK, et al. EAU guidelines on surgical treatment of urinary incontinence. Actas Urol Esp 2013;37:459-72. PMID: 23835037


Fig. 1.
Receiver operating characteristic curves of forward and backward models. AUC, area under the curve.
Table 1.
Prevalence of SUI in MS by age range
| Age range (yr) | No. | Prevalence of SUI in MS (%) |
|---|---|---|
| 18–24 | 5 | 60.0 |
| 25–29 | 15 | 20.0 |
| 30–34 | 26 | 26.9 |
| 35–39 | 43 | 44.2 |
| 40–44 | 75 | 30.7 |
| 45–49 | 56 | 23.2 |
| 50–54 | 55 | 36.4 |
| 55–59 | 39 | 25.6 |
| 60–69 | 44 | 34.1 |
| ≥ 70 | 5 | 20.0 |
Table 2.
Clinical characteristics of MS patients
| Variable | SUI (n=114) | No SUI (n=249) | P-value |
|---|---|---|---|
| Disease duration at the time of urodynamic exam | 11(10) | 12 (11) | 0.270 |
| Clinical form (%) | 0.017* | ||
| PPMS | 6.1 | 6.8 | |
| SPMS | 22.8 | 37.4 | |
| RRMS | 71.1 | 55.8 | |
| EDSS score | 4 ⑶ | 5.5 (3) | 0.030* |
| Treatment for MS (%) | 0.324 | ||
| None | 45.6 | 53.4 | |
| Immunomodulator | 25.4 | 19.3 | |
| Immunosuppressor | 18.4 | 20.1 | |
| Monoclonal antibodies | 10.5 | 7.2 | |
| Urological treatment (%) | |||
| Anticholinergic | 21.9 | 16.1 | 0.228 |
| Alpha-antagonist | 9.6 | 16.1 | 0.142 |
Table 3.
Clinical characteristics of multiple sclerosis form of patients
| Variable | PPMS (n=24) | SPMS (n=119) | RRMS (n=220) | P-value |
|---|---|---|---|---|
| Age (yr) | 48.5 (10.4) | 51 (14.0) | 42.5 (14.0) | <0.001* |
| EDSS score | 6 (2.3) | 6.5 (1.0) | 4 (2.1) | <0.001* |
| Disease duration at the time of urodynamic exam (yr) | 5.5 (7.0) | 17 (11.0) | 10 (10.0) | <0.001* |
Table 4.
Low urinary tract symptoms in patients with multiple sclerosis
| Variable | SUI (n=114) | No SUI (n=249) | P-value |
|---|---|---|---|
| POP (%) | 4.4 | 1.6 | 0.146 |
| Constipation (%) | 39.5 | 35.3 | 0.521 |
| Anorectal dyschesia (%) | 7.9 | 7.6 | 1.000 |
| Urinary infections (%) | 21.9 | 28.5 | 0.233 |
| Pyelonephritis (%) | 3.5 | 3.2 | 1.000 |
| Self-catheterization (%) | 2.6 | 9.6 | 0.017* |
| No. of daily incontinent episodes | 1.3±1.7 | 0.7±1.3 | <0.001* |
| Drink (L/day) | 1.4±0.4 | 1.4±0.5 | 0.565 |
| Pollakisuria (%) | 42.1 | 41.0 | 0.928 |
| Nocturia (%) | 25.4 | 23.7 | 0.820 |
| Voiding dysfunction (%) | 48.2 | 63.0 | 0.011* |
| Urgency (%) | 57.9 | 58.6 | 0.985 |
| Overflow incontinence (%) | 7.0 | 11.6 | 0.243 |
| Urge urinary incontinence (%) | 60.5 | 43.0 | 0.003* |
Table 5.
Urodynamic investigations in patients with multiple sclerosis
| Variable | Total population (n=363) | SUI (n=114) | No SUI (n=249) | P-value |
|---|---|---|---|---|
| Cystometry | ||||
| Initial detrusor pressure (cm H2O) | 15.9±8.8 | 17.1±9.5 | 15.3±8.5 | 0.076 |
| NA (n) | 2 | 1 | 1 | |
| Bladder sensation (%) | 0.623 | |||
| Oversensitivity | 26.7 | 24.6 | 27.7 | |
| Reduced bladder sensation | 33.1 | 31.6 | 33.7 | |
| Normal sensation | 40.2 | 43.9 | 38.6 | |
| Detrusor activity (%) | 0.043* | |||
| Normal | 62.5 | 71.9 | 58.2 | |
| Detrusor overactivity | 30.9 | 22.8 | 34.5 | |
| Detrusor underactivity | 6.6 | 5.3 | 7.2 | |
| Maximum bladder capacity (mL) | 432.7±187.3 | 414.5±171.6 | 441±193.8 | 0.212 |
| Bladder compliance (%) | 0.841 | |||
| Normal | 51.2 | 51.8 | 51 | |
| Decreased | 32.5 | 30.7 | 33.3 | |
| Increased | 16.3 | 17.5 | 15.7 | |
| Strong desire to void (mL) | 412±176.5 | 394.2±161.4 | 420.5±183 | 0.361 |
| NA (n) | 32 | 7 | 25 | |
| Urethral pressure | ||||
| MUCP | 70±33 | 65.2±34 | 72.2±32.3 | 0.031* |
| NA (n) | 7 | 1 | 6 | |
| Sphincter deficiency (%) | 0.300 | |||
| Yes | 1.4 | 0.8 | 1.6 | |
| No | 96.7 | 98.3 | 96.0 | |
| NA | 1.9 | 0.9 | 2.4 | |
| During straining (%) | <0.001* | |||
| Uninterpretable | 24.5 | 10.5 | 30.9 | |
| Impossible | 28.1 | 41.2 | 22.1 | |
| Poor quality | 17.9 | 26.3 | 14.1 | |
| Possible | 29.5 | 21.9 | 32.9 | |
| Uroflowmetry | ||||
| Maximum flow rate (mL/sec) | 24.3±11.7 | 27±10.2 | 22.9±12.1 | <0.001* |
| NA (n) | 82.0 | 21.0 | 61.0 | |
| Postvoid residual (%) | 0.004* | |||
| Significant | 20.1 | 10.5 | 24.5 | |
| No significant | 60.3 | 71.1 | 55.4 | |
| Uninterpretable | 19.6 | 18.4 | 20.1 | |
| Aspect of curvea) (%) | 0.005* | |||
| Flat | 14.3 | 9.6 | 16.5 | |
| Cloche | 45.2 | 58.8 | 39.0 | |
| Chopped | 17.9 | 13.2 | 20.1 | |
| Uninterpretable | 22.6 | 18.4 | 24.5 | |
| Catheterization | 16.0 | 7.9 | 19.7 | 0.007* |
| Detrusor sphincter dyssynergia (%) | 5.2 | 2.6 | 6.4 | 0.203 |
Values are presented as mean±standard deviaion unless otherwise indicated.
SUI, stress urinary incontinence; NA, not applicable; MUCP, maximal urethral closure pressure.
Table 6.
Results of logistic regression with backward model of risk factors of stress urinary incontinence in patients with multiple sclerosis