INTRODUCTION
Overactive bladder (OAB) is a clinical syndrome defined by the International Continence Society as “urgency, with or without urge incontinence, usually with frequency and nocturia, in the absence of other underlying pathology, including urinary tract infection (UTI)” [1]. OAB is a prevalent urinary disorder with a major impact on daily activities and quality of life. It is estimated that OAB affects the urinary health of 12% to 17% of the total population, and its prevalence increases with age [2,3]. The causes of OAB may be neurogenic, myogenic, urotheliogenic, or integrative [4,5].
Patient education and behavioral therapy are recommended as the first-line standard treatments for OAB. Subsequently, pharmacologic treatments, including antimuscarinic agents or β3-agonists, are administered [6]. However, many patients cannot adhere to conservative treatments owing to dissatisfactory efficacy or intolerable side effects such as dry mouth and constipation. When the previously mentioned treatments are insufficient, several minimally invasive surgical techniques can be considered, such as intravesical botulinum toxin type A (BoNTA) injection, posterior tibial nerve stimulation, and sacral neuromodulation. As a last resort, specialized surgical treatments, including augmentation cystoplasty and urinary diversion, are available for refractory urgency urinary incontinence induced by OAB.
Botulinum toxin is a neurologically destructive neurotoxin synthesized by the bacterium Clostridium botulinum [7]. Seven subtypes of botulinum toxin (A-G) are available for clinical use. Given its long effect duration, BoNT-A is used more often than the other botulinum toxin subtypes in urology. Because the molecular weight of BoNT-A is 150 kDa, it cannot access the submucosal nerve plexus in solution. Clinically, BoNT-A is directly injected, bypassing the urothelium to achieve botulinum toxin administration. However, BoNT-A injection therapy has many common and bothersome adverse events (AEs), such as leakage or uneven distribution of the drug, hematuria, pain, and risk of infection [8,9]. The incidence of these AEs varies among the results of many trials. Kuo et al. [10] reported that the most common AEs after botulinum toxin injection in 217 patients with refractory idiopathic detrusor overactivity (DO) were straining to void (46.5%), large postvoid residual (PVR) >150 mL (47.5%), UTIs (14.3%), and gross hematuria (7.8%). In a systematic review regarding intradetrusor Botox injections in patients with neurogenic DO, the most common reported AEs were injection site pain, UTIs (2%–32%), mild hematuria (2%–21%), and increased PVR, potentially resulting in urinary retention (0%–33%) or de novo intermittent self-catheterization (6%–88%) [11]. The diffusion of botulinum toxin beyond the injection site is also linked to a variety of life-threatening complications, such as generalized muscle weakness, dysphagia, and breathing difficulties, which are observed in rare cases after lower urinary tract injections of botulinum toxin [12,13]. Therefore, a safe and effective botulinum toxin preparation or delivery method to replace the traditional direct injection is urgently needed.
Nanoparticles, materials with a scale of 1 to 1,000 nm, have been developed rapidly in recent years. Nanomaterials are versatile in several research fields because of their characteristics, including a high surface-area-to-volume ratio, flexible construction, good biocompatibility, and biodegradability [14]. Nanomedicine, which incorporates different medical disciplines, has emerged as an especially promising discipline in the field of drug delivery. Advancements in intravesical drug delivery (IDD) systems have been significant for the treatment of urinary system diseases. Dose reduction increases drug effectiveness by targeting the site of the drug’s action. The other advantages of IDD include uniform delivery of the active agent, maximization of the therapeutic effect, and minimization of systemic side effects [15,16]. Specifically, compared to BoNT-A injection, the development of IDD as a method of BoNT-A administration for patients could simplify the treatment procedure by avoiding the need for anesthesia and cystoscopy and thus drastically reduce the treatment costs for patients with refractory OAB. More importantly, this procedure may improve AEs. However, the efficacy of intravesical delivered BoNT-A is restricted by its residence time and the extent to which it attaches to the bladder wall and penetrates into it. Researchers have tried to develop penetration enhancers to improve efficacy to some extent by using physical or chemical methods, such as electromotive force, chitosan, and dimethyl sulfoxide, which have yet to be optimized for large-scale use [17].
Interest has recently increased in intravesically administered agents carried by nanostructured systems, which can provide effective and safe therapy for bladder disorders. Therefore, in this review, we discuss recent developments in nanostructured IDD systems, the most recent formulation technologies, and the feasibility and physicochemical characteristics of the systems, providing prospects for progress in this field.
BLADDER PERMEABILITY BARRIER
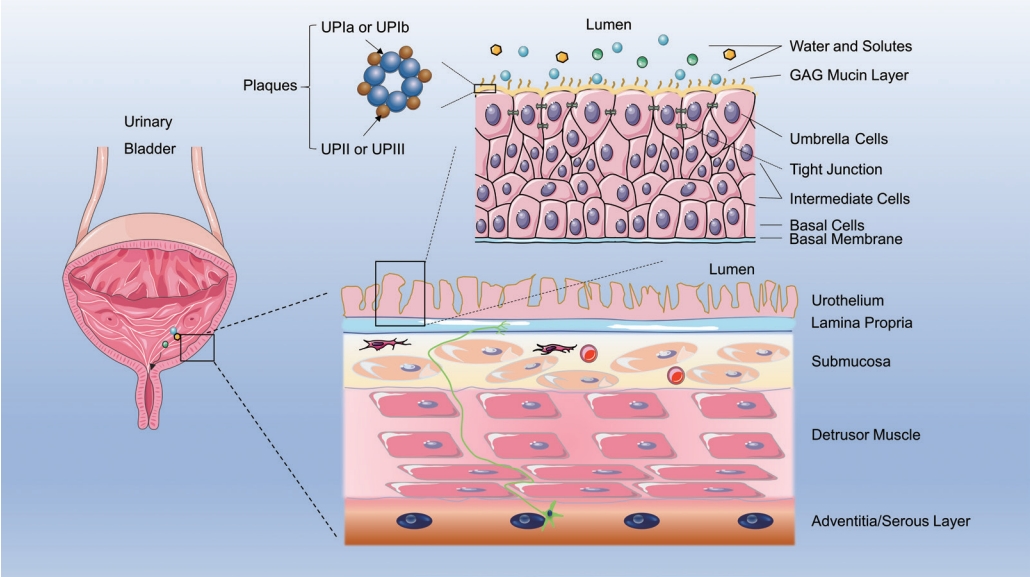
The 4 layers of the bladder wall, from inside to outside, are the mucosa, the submucosa consisting of lamina propria and muscularis mucosae, the muscular layer consisting of the detrusor, and the serous layer [18]. As the main component of the bladder mucosal layer, the transitional urothelium acts as a physiological barrier between components of urine and the submucosal layers [19]. The urothelium is comprised of 3 layers of cells, including basal cells, intermediate cells, and the characteristically shaped surface umbrella cells. Tight junctions connecting the umbrella cells, densely packed plaques, and the glycosaminoglycan (GAG)-mucin layer form the bladder permeability barrier (BPB) (Fig. 1) [20].
The BPB is significant for the design of effective IDD. The bladder epithelium is regenerated by the differentiation of basal cells into the intermediate layer, with subsequently differentiation into the superficial epithelial layer, which contains polarized umbrella cells [21]. The diameter of umbrella cells can change from 50 to 120 μm, according to the degree of bladder expansion. The tight junctions connecting umbrella cells may reduce the permeability of ions, waste solutes, and some lipids [22], while the plaques covering the apical membrane can block small molecules such as water and urea. The hexagonal plaques consist of 4 uroplakins arranged in an orderly manner: UPIa (27 kDa), UPIb (28 kDa), UPII (15 kDa), and UPIII (47 kDa) (Fig. 1) [23,24].
The GAG layer serves as an antiadherence and anti-infection barrier on the urothelial surface, preventing solutes from reaching underneath the tight junctions and cell membranes [25,26]. The submucosal layer is made up of interstitial cells, blood vessels, myofibroblasts, and afferent sensory nerve endings in the lamina propria, tightly arranged under the basal membrane (Fig. 1). Meanwhile, the BPB structure of the urothelium restricts the adhesion and penetration of drugs after intravesical delivery, leading to elimination of the drug’s active ingredient. Hence, many drugs fail to reach their site of action in the bladder and ultimately cannot achieve the desired therapeutic effects.
MECHANISM OF ACTION OF BoNT-A
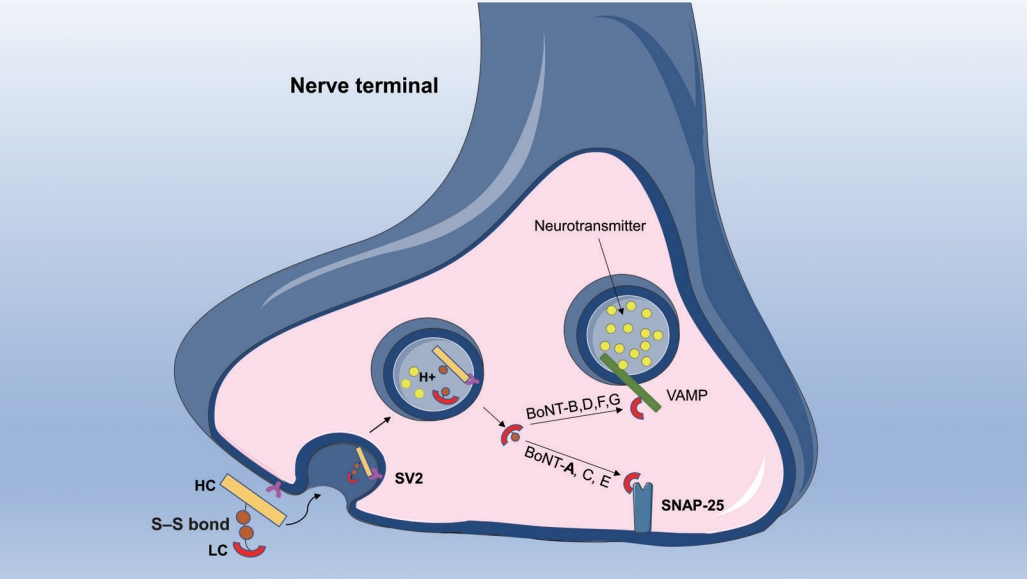
Structurally, BoNT-A consists of a 50-kDa light chain and a 100-kDa heavy chain linked by a noncovalent disulfide bond [27]. The process of BoNT-A entering the cell begins with recognition by 2 types of receptors on the cell membrane surface: gangliosides and synaptic vesicle glycoprotein 2 (SV2). Recent studies have also predicted that fibroblast growth factor receptor 3 may be an alternative receptor for BoNT-A [28]. The heavy-chain C-terminal of BoNT-A recognizes and binds to SV2 on the synaptic membrane of the nerve terminals, and BoNT-A is internalized into the nerve terminal by endocytosis [29].
After being transported into the cytosol, the disulfide bond of BoNT-A is cleaved in the synaptic vesicle. The N-terminal of the heavy chain binds to the endosomal membrane, and the light chain is transported from the endosome to the cytosol as the true active moiety of BoNT-A. Then the light chain cleaves synaptosome-associated protein 25 (SNAP-25), blocking the function of the soluble N-ethylmaleimide-sensitive factor attachment receptor family, which is essential for the transport of vesicles and signal transduction. Hence, BoNT-A inhibits neurotransmitter release by disrupting the fusion of vesicles with the neuron cell membrane, paralyzing the affected neuromuscular junctions (Fig. 2) [29].
Previous research has confirmed the distribution of SV2 and SNAP-25 in the submucosa and detrusor, but with no expression in the urothelium [30]. SV2 and SNAP-25 are expressed more abundantly in cholinergic than in parasympathetic fibers, with less than 50% of expression in the sensory and sympathetic nerves. Another study has demonstrated that the cleaved SNAP-25 is predominantly expressed in the parasympathetic nerve and that botulinum toxin mainly cleaves the SNAP-25 protein in cholinergic fibers [31]. These observations indicate that the parasympathetic nerves are the main target of BoNT-A action in the human bladder and that cleaved SNAP-25 is considered an important marker of sites of BoNT-A action for future research [30,31]. Other clinical studies and animal models have demonstrated that SV2 and SNAP-25 are expressed in the urothelial cells and mucosa of the rat and human bladder [32,33]. Because the urothelium is also an important signal transducer [18], many findings indicate that the IDD of BoNT-A may exert a therapeutic effect by acting on the urothelium through inhibition of neurotransmitter release from urothelial cells [34,35].
After BoNT-A is injected into the detrusor, the release of acetylcholine at the neuromuscular junction is temporarily blocked, and the actions of α and γ motor neurons innervate the extrafusal and the intrafusal muscle fibers, respectively. A randomized controlled trial on the effect of onabotulinum toxin A detrusor injections on postsynaptic muscular receptors in the human bladder with neurogenic DO showed downregulation of M2, M3, P2X2, and P2X3 receptors in the detrusor muscle after onabotulinum toxin A injections [36]. Traditionally, the therapeutic effects of BoNT-A on OAB have been attributed to the inhibition of detrusor or urethral sphincter contractions, suggesting that BoNT-A inhibits DO by affecting sensory and motor signal transmission.
Recent studies have revealed that BoNT-A injections can inhibit bladder sensory nerve function by reducing the expression of different receptors on afferent nerve fibers, including the ATP receptor P2X3 and the transient receptor potential vanilloid subfamily-1 (TRPV1) [37]. The decrease of TRPV1 and P2X3 immunoreactivity is correlated to fewer urgency episodes [37]. Other studies have shown that intravesical BoNT-A injections significantly inhibited the release of ATP and neurotrophin while increasing the release of nitric oxide from the urothelium [35,38].
NANOTECHNOLOGY IN INTRAVESICAL DRUG DELIVERY OF BoNT-A
Many studies have shown that direct intravesical instillation of BoNT-A cannot exert therapeutic effects [31,39]. Some factors prevent BoNT-A from penetrating the mucosa: First, the passive diffusion of BoNT-A is limited by tight junctions due to its large molecular weight (150 KDa). Second, the intact BPB blocks the adherence and permeation of BoNT-A, and third, the dilution and flushing effect of urine prevents BoNT-A from remaining localized in the bladder [40]. As previously mentioned, BoNT-A injections are associated with AEs in patients. Therefore, urologists have tried to overcome these obstacles with different approaches. The goal of these studies has been to achieve deep penetration of BoNT-A into the bladder and sufficient residence time in the bladder.
Currently, IDD, combined with nanotechnological approaches, is a potential tool to improve BoNT-A therapies. Nanomaterials, categorized into carbon-based materials (e.g., carbon nanotubes, carbon nanofibers, graphene), inorganic-based materials (e.g., gold, titanium oxide, silicon), organic-based materials (e.g., dendrimers, micelles, liposomes, polymer nanoparticles), and composite-based materials, have versatile properties as nanocarriers that enable penetration or retention in many tissues, including the urothelium [41,42]. Several nanoparticle formulations of BoNT-A have been investigated to improve IDD (Fig. 3).
Liposome Formulation of BoNT-A
Liposomes are spherical vesicles made up of an aqueous core surrounded by 1 or more phospholipid layers. Liposomes can load both lipophilic drugs (e.g., capsaicin) and hydrophilic drugs (e.g., botulinum toxin) [43,44]. Subsequently, liposomes penetrate the urothelium via endocytosis for IDD [45]. Empty liposomes have been shown to partially reverse high micturition frequency in a rat model of bladder hyperactivity induced by potassium chloride irritation after protamine sulfate intravesical instillation [46]. This indicates that empty liposomes may enhance the BPB by forming a protective lipid film on the urothelial surface against irritant penetration. Treatment with liposomes alone had no toxic effects on the urothelium. Liposomes were also found to protect urothelial cells from acrolein damage [47].
In a rat model of acetic acid-induced bladder hyperactivity, Chuang et al. [44] demonstrated that liposome-encapsulated BoNT-A significantly reduces bladder overactivity. Rats that received liposome-encapsulated BoNT-A (lipotoxin) had a larger decrease in the intercontraction interval (ICI) response to acetic acid instillation than liposome- or BoNT-A–pretreated rats, without compromising voiding function. Furthermore, intravesical lipotoxin instillation can cleave SNAP-25, inhibiting the release of calcitonin gene-related peptide from afferent nerves. The results indicate that BoNT-A with liposome as a vehicle can effectively and safely cross the BPB without a direct injection. Similar results were observed in animal studies with liposomes encapsulating capsaicin [43].
Furthermore, Kuo et al. [33] reported a double-blind randomized controlled pilot trial in 24 OAB patients at a single center to assess the safety and efficacy of lipotoxin. In this study, lipotoxin-containing 80-mg liposomes and 200 U of BoNT-A or normal saline were intravesically instilled and voiding diaries, Overactive Bladder Symptom Scores, urodynamic parameters, and AEs were assessed. At 1 month posttreatment, urinary frequency every 3 days and urgency significantly improved in the lipotoxin treatment group when compared to the control group. Taken together, the results showed no increase in PVR or risk of UTI in the lipotoxin-treated group. Immunohistochemistry and western blot studies showed the expression of SV2 and SNAP25 in urothelial cells and suburothelial tissues. However, no differential expression of SV2 or SNAP25 was observed between responders and nonresponders 3 months after treatment.
In a subsequent 2-center, placebo-controlled trial using the same lipotoxin, Chuang et al. [48] enrolled 62 participants with OAB inadequately managed with antimuscarinics to investigate whether intravesical instillation of lipotoxin could significantly decrease OAB symptoms and urinary frequency every 3 days without the AEs of dysuria, increased PVR, or UTI. However, lipotoxin treatment did not change the frequency of urgency urinary incontinence episodes in the 2 clinical trials. We could infer from the trials and the animal model that the penetration depth of intravesically instilled lipotoxin is not as deep as that of detrusor injections. Future studies could help determine whether the action of lipotoxin is restricted to the urothelium or lipotoxin is further transported to the submucosa for action. A larger randomized controlled trial is also needed to validate the effectiveness of lipotoxin in OAB.
Other studies have considered the application of lipotoxin for bladder sensory disorders such as interstitial cystitis/bladder pain syndrome (IC/BPS). However, Chuang et al. [49] demonstrated that the therapeutic effect of a single intravesical instillation of lipotoxin for patients with moderate to severe IC/BPS was likely due to a significant placebo effect. A study with a rat model of ketamine-induced cystitis showed that repeated intravesical instillation of lipotoxin can ameliorate bladder overactivity in ketamine-treated rats and restore the urothelial tight junction and adhesion proteins [50].
Thermosensitive Hydrogel Delivery of BoNT-A
Thermosensitive polymeric hydrogel stays in a liquid state at room temperature or below but converts to a semisolid state at a higher temperature (e.g., body temperature [37°C]). Thus, it can be injected in its liquid state and forms a hydrogel in situ in the bladder at higher body temperatures [51]. The triblock copolymer poly(ethylene glycol)-poly(lactic acid-co-glycolic acid)-poly(ethylene glycol) was used to form a polymeric hydrogel, which can be modified to acquire more hydrophobic characteristics and withstand urine components [52]. Because the hydrogel has the advantages of easy synthesis, good biocompatibility, and biodegradability, it can be used as an effective nanocarrier for IDD to maintain a prolonged release of drugs to the urothelium.
A double-blind randomized pilot study evaluated the effect of BoNT-A embedded in an inert TC-3 hydrogel in patients with idiopathic OAB [53]. The results revealed that intravesical instillation of 200 U BoNT-A in 50 mL of TC-3 gel can decrease urgency and leakage episodes and improve Overactive Bladder Questionnaire and Patient Perception of Bladder Condition total scores in patients with OAB after 1 month of treatment. Rappaport et al. [54] conducted a pilot study in 2018 to evaluate the feasibility and safety of a single intravesical instillation of 200 U of BoNT-A in 40 mL of TC-3 gel for IC/BPS patients. The study reported that intravesical instillation of BoNT-A could reduce the Visual Analog Scale for pain, Interstitial Cystitis Symptom Index, and Interstitial Cystitis Problem Index at week 12 compared with baseline, with transient and mild AEs. However, there was a borderline therapeutic effect on nocturia and urge episodes upon returning to baseline at week 12.
Hyaluronan-Phosphatidylethanolamine Delivery of BoNT-A
Ubiquitously distributed in biological fluids and tissues, hyaluronic acid (hyaluronan, HA) is a hydrophilic polysaccharide formed from disaccharide units containing N-acetyl-D-glucosamine and glucuronic acid. It has interesting rheological, viscoelastic, and biological properties, which are attributed to its polymeric and polyelectrolyte characteristics [55-57]. Topically applied HA has poor penetration for its molecular weight of 50 kDa. Thus, linking HA to phosphatidylethanolamine (PE) to develop a nonparticulate formula can enhance its penetrating properties and HA levels throughout epidermal cell layers [58]. Its high viscosity makes HA-PE an excellent carrier for transferring BoNT-A through the urothelium.
In 2017, El Shatoury et al. [59] examined the effect of intravesical instillation of BoNT-A embedded in HA-PE in a rat model of hyperactive bladder induced by 1% acetic acid instillation. The results proved that bladder instillation of 10 U BoNT-A embedded in 0.4 or 0.5 g of HA-PE for 60 minutes, instead of BoNT-A with a lower HA-PE dose or a shorter instillation time, transferred BoNT-A throughout the bladder urothelium. In this study, both HA-PE–embedded and intradetrusor-injected BoNT-A showed comparable cleavage of SNAP-25, and rats in both groups showed a comparable effect on the prolongation of ICI compared with baseline. These results all suggested that adequate HA-PE with a sufficient instillation time favors the ability of BoNT-A embedded in HA-PE to penetrate the bladder mucosa and reach comparable efficacy as intradetrusor injection.
OTHER NANOCARRIERS FOR INTRAVESICAL DRUG DELIVERY
Nanotechnology in IDD is not only applied to lower urinary tract dysfunction, but is widely used in chemotherapy and bacillus Calmette-Guérin immunotherapy against bladder cancer. The nanocarriers developed for bladder cancer intravesical therapy were mainly based on microparticles and nanoparticles, carbon nanotubes, hydrogels, nanogels, liposomes, and micelles [60]. These nanocarriers can transport the active agent of drugs to the target sites and provide controlled release, and their biocompatible and biodegradable natures enable them to be used in IDD [61-65]. Currently studied nano-scale materials with great potential include chitosan, dendrimers, and lipid, protein, polymeric, magnetic, and inorganic nanoparticles [16,20,66,67]. For their excellent mucosal adhesion and penetration characteristics, they may be able to be used as a carrier of botulinum toxin to achieve safe and effective submucosal administration. However, different drugs have distinct physicochemical and pharmacodynamic characteristics, which determine their unique clinical efficiency and side effects [68]. Given the pathological differences between bladder cancer and OAB, ongoing experiments are needed to verify the feasibility of these nanocarriers.
CONCLUSIONS AND FUTURE PROSPECTS
Nanotechnology in IDD, directing the instillation of botulinum toxin with nanocarriers into the urinary bladder via catheters, is an effective alternative to intradetrusor injections. This method, which can maximize local therapeutic effects while minimizing adverse effects, relies on the development of nanocarriers that can effectively penetrate the urothelial barrier to reach the submucosa and deeper layers, stably release drugs, and be safe to the bladder tissue. In this review, suitable nanocarriers for transferring BoNT-A into the bladder were discussed. The effectiveness of liposome-encapsulated BoNT-A has been investigated in animal models and clinical trials. However, intravesical instillation of lipotoxin did not reach a similar efficacy as intradetrusor injection, and the therapeutic effect of lipotoxin lasted only 1 month. Clinical trials of thermosensitive hydrogelembedded BoNT-A were only marginally satisfactory, and clinical trials of HA-PE are needed to prove its efficacy. However, each type of nanocarrier has specific disadvantages, and it is difficult to decide which nanocarrier would be most promising. Research on liposomes is the earliest and most detailed, yet the potential of other nanocarriers should not be dismissed. Further research is required to discover potential nanocarriers with excellent mucosal adhesion and penetration abilities for intravesical instillation-based therapy. We hope that these safe, efficient carriers can be developed and become clinically available to patients with bladder dysfunction in the near future.