 |
 |


- Search
| Int Neurourol J > Volume 26(3); 2022 > Article |
|
ABSTRACT
Purpose
This study investigated functional outcomes in lower urinary tract symptoms (LUTS), the incidence of incidental prostate cancer (PCa), and changes in prostate-specific antigen (PSA) levels after holmium laser enucleation of the prostate (HoLEP) in patients with elevated PSA and benign prostatic hyperplasia (BPH).
Methods
A retrospective review of a prospectively designed protocol for patients who underwent HoLEP at our institution from January 2010 to May 2020 was conducted. Patients were classified into low-PSA (<3.0 ng/mL) and high-PSA (≥3.0 ng/mL) groups at baseline. Follow-up for PSA was performed at the sixth postoperative month. Baseline and postoperative clinical parameters, functional parameters, PCa incidence, and postoperative changes in PSA were compared between the low- and high-PSA groups.
Results
The baseline PSA of 1,296 patients (mean age, 69.7±6.8 years) was 4.0±4.1 ng/mL, with 712 patients (55.0%) in the low-PSA group (1.6±0.8 ng/mL), and 584 patients (45.0%) in the high-PSA group (6.9±4.7 ng/mL). Incidental PCa was detected in 82 patients (6.3%), with a similar incidence in the low-PSA (41 patients, 5.9%) and high-PSA (41 patients, 7.0%) groups (P>0.05). At 6 months postoperatively, both groups showed significant improvements in the maximum flow rate, postvoid residual volume, and all domains of the International Prostate Symptom Score (P<0.05). At postoperative 6 months, the PSA level significantly decreased by 66.6%±23.6% in all patients (54.3%±23.9% in the low-PSA group; 79.6%±14.7% in the high-PSA group) (P<0.05), and the PSA levels of 1,264 patients (97.6%) had normalized.
Conclusions
In patients with elevated PSA presenting with LUTS/BPH, our study demonstrated significant improvements in functional parameters and decreased PSA after HoLEP. The incidental PCa detection rate did not show a statistically significant difference between the low- and high-PSA groups. Timely surgery for LUTS/BPH without delay due to PSA monitoring should be considered.
Benign prostatic hyperplasia (BPH) is becoming increasingly common with population aging, and more men presenting with lower urinary tract symptoms (LUTS) caused by BPH are seeking treatment [1]. Upon screening, prostate-specific antigen (PSA) elevation is often observed in patients with BPH, which is sometimes difficult to differentiate from the PSA elevation observed in prostate cancer (PCa) patients [2].
Managing patients with bothersome LUTS and persistently elevated PSA is a modern urologic dilemma when making clinical decisions [3]. For patients with persistent PSA elevation, urologists have a tendency to conduct continuing PSA monitoring and multiple additional prostate biopsies (P-Bx) for PCa evaluation over a long period of time. This can delay optimal surgical treatment, prolong LUTS, and decrease the quality of life (QoL) [4]. Several algorithms have been proposed to deal with this situation, without a clear consensus [5].
Limited studies have evaluated the clinical outcomes of transurethral resection of the prostate (TURP) in patients with LUTS and elevated PSA. Cho et al. [6] reported that in patients with BPH and elevated PSA, TURP improved functional outcomes and reduced PSA levels. Tinmouth et al. [7] also analyzed the importance of PSA as an objective tool for evaluating BPH treatment. However, a more detailed understanding of the outcomes of patients with PSA elevation and LUTS receiving holmium laser enucleation of the prostate (HoLEP) could assist in clinical decision-making for timely surgical treatment.
Our clinical experiences suggested that timely surgical treatment of patients with elevated PSA and BPH without a delay due to PSA follow-up could show comparable clinical outcomes to those of patients without PSA elevation. We hypothesized that there would be significant differences between low- and high-PSA groups in terms of symptom improvement and the incidence of incidental PCa detection.
This study reviewed a patient cohort at our institution from January 2010 to May 2020. The Institutional Review Board (IRB) of the Seoul National University Hospital approved this study (IRB No. H-2111-182-1277). The same clinical pathway under a prospectively designed study protocol for BPH surgery, including preoperative and postoperative follow-up evaluations and the timeline thereof, was applied to all surgical patients after 2009 at Seoul National University Hospital. With treatment optimization in mind, an order set was made in the electronic medical record system so that the same work-up was applied to all eligible patients. Data from the patient group following a prospectively designed patient study protocol were analyzed retrospectively.
The inclusion criteria included patients aged ≥50 years with a clinical diagnosis of BPH who underwent HoLEP. The exclusion criteria were patients who had a history of genitourinary cancer and pelvic surgery, and neurogenic lower urinary tract dysfunction. Patients with minimal neuropathy, such as transient ischemic attack, which had little effect on LUTS as judged by a history and physical examination, were included in this study. The PSA level (Roche Diagnostics, Indianapolis, IN, USA) was measured at both baseline and postoperatively. For patients whose PSA was elevated at baseline, a meticulous screening work-up was conducted to eliminate cancer risk in patients who were indicated for prostatectomy for BPH. Urinalyses were conducted for all patients to detect urinary tract infections (UTIs), which were treated if diagnosed, and the patients were followed up until no evidence of infection was detected. Medications for LUTS, the number of acute urinary retention (AUR) episodes, the International Prostate Symptom Score (IPSS) [8], total prostate volume (TPV) and transitional zone volume (TZV) measured using transrectal ultrasonography, the number of transrectal P-Bx, uroflowmetry parameters, and urodynamic parameters were evaluated as previously described in the literature [9]. P-Bx was considered when the PSA level was high (≥3.0 ng/mL), when a digital rectal exam showed suspicious findings, and/or when imaging showed abnormal findings [10]. For patients taking 5-alpha reductase inhibitors (5-ARIs), the PSA value was multiplied by 2 [11], and this doubled value was used to determine whether to perform a P-Bx. At our institution, until 2017, 12-core biopsies were conducted near the base, midgland, and apex. After 2018, 14-core P-Bx including both transitional zones were conducted [12,13].
HoLEP was conducted using the technique we previously described [14]. The intraoperative parameters that were evaluated were operative time, energy use, the weight of enucleated prostate tissue, and morcellation time. After surgery, all BPH medications were discontinued. If patients experienced overactive bladder symptoms postoperatively, anticholinergics and/or beta-3 agonists were prescribed. In patients who were diagnosed with PCa based on surgical pathology, the pathological results followed the Gleason staging system [15], the tumor volume percentage was recorded, and they were referred to urologic oncologists.
Postoperative parameters were collected and complications were evaluated at postoperative 2 weeks, 3 months, and 6 months, as previously described in the literature [16]. In principle, 6 months after surgery, open follow-up was conducted. Follow-up was extended and individualized for a small number of patients who showed abnormal findings during follow-up, such as persistently elevated PSA or pyuria, 6 months after surgery.
The patients were classified into low-PSA (<3.0 ng/mL) and high-PSA (≥3.0 ng/mL) groups. The preoperative parameters, perioperative parameters, and postoperative parameters were compared between the low- and high-PSA groups. The statistical analyses were conducted using IBM SPSS Statistics ver. 22.0 (IBM Co., Armonk, NY, USA). We expressed continuous variables as the mean±standard deviation. Differences between the low- and high-PSA groups were analyzed with the Student t-test for continuous data, and the chi-square test for categorical values. The baseline characteristics, IPSS score changes, and operative pathology of the low- and high-PSA groups were analyzed. A P-value of <0.05 was considered to indicate statistical significance, which means that the null hypotheses mentioned in the Introduction could be rejected. Multivariable regression analysis was used to analyze the factors showing correlations with baseline PSA. The correlations between the diagnosis of incidental PCa after HoLEP and PSA, P-Bx, and 5-ARI were investigated, and the low- and high-PSA groups were compared.
The baseline characteristics of 1,296 consecutive patients are shown in Table 1. The average age of the patients was 69.7±6.8 years. The mean total IPSS was 19.8±10.5, and the mean TPV was 68.3±33.6 mL. In total, 294 patients (22.7%) experienced more than one AUR episode, and 442 patients (34.1%) underwent more than one P-Bx. The baseline PSA of all patients was 4.0 ±4.1 ng/mL, with 712 (55.0%) in the low-PSA group (1.6 ±0.8 ng/mL), and 584 (45.0%) in the high-PSA group (6.9±4.7 ng/mL). The body mass index (BMI), postvoid residual volume, number of preoperative AURs, baseline PSA, TPV, TZV, the number of preoperative P-Bx, and bladder outlet obstruction (BOO) all were statistically significantly different between the low- and high-PSA groups at baseline (P<0.05).
The changes in all patients for PSA and objective and subjective storage and emptying parameters compared to baseline are shown in Table 2. There were no significant differences between the low- and high-PSA groups at baseline, nor between the low- and high-PSA groups in terms of symptom score improvement as calculated by the changes in baseline scores at the postoperative third and sixth months (P>0.05). However, at the postoperative third and sixth months, there were significant differences between the low- and high-PSA groups in terms of the IPSS-obstructive symptom score, IPSS-total score, and IPSS-QoL score (P<0.05). All functional parameters at the sixth postoperative month showed statistically significant improvements compared with baseline (P<0.05) (Supplementary Table 1).
The surgical pathology after HoLEP is described in Table 3. Eighty-two patients (6.3%) were diagnosed with incidental PCa after HoLEP, with a similar distribution between the low-PSA (41 patients, 5.9%) and high-PSA (41 patients, 7.0%) groups. The hypothesis that there would be a difference in the incidence of incidental PCa detection between the low- and high-PSA groups was rejected since the chi-square test for homogeneity showed no statistical significance (P >0.05). Most of the patients had a tumor volume of 1% in both the low- and high-PSA groups. The total Gleason score and tumor percentage were likewise not statistically different between the low- and high-PSA groups (P>0.05). PCa was not significantly correlated with the baseline PSA level (P =0.25), number of P-Bx (P=0.95), or 5-ARI use (P=0.25), and these findings were consistent in both the low- and high-PSA groups.
The change in PSA from baseline to postoperative 6 months was analyzed (Fig. 1). The PSA level (4.0±4.1 ng/mL at baseline) decreased to 1.0±0.9 ng/mL at postoperative 6 months (PSA reduction, 66.7%±23.6%). This decrease was similar in the low-PSA group (PSA reduction, 54.3% ±23.9%), which showed a drop from 1.6±0.8 ng/mL at baseline to 0.8±0.5 ng/mL. In the high-PSA group, the PSA level decreased more significantly, from 6.9±4.7 ng/mL at baseline to 1.2±1.1 ng/mL (PSA reduction, 79.6%±14.7%) at 6 months postoperatively. These changes were all statistically significant when the 6-month value was compared to baseline (P<0.005). Among the patients with baseline low PSA (n=712), 709 (99.6%) normalized postoperatively, and 3 (0.4%) were de novo postoperatively elevated (≥3.0 ng/mL). Among the patients with baseline high PSA (n =584), 556 (95.2%) normalized, and 28 (4.8%) were postoperatively persistently elevated (≥3.0 ng/mL). The PSA decrease rate showed a statistically significant correlation with the resected volume (P<0.001).
The high-PSA group showed an increased risk of AUR (≥1 time) (odds ratio [OR], 2.39; 95% confidence interval [CI], 1.82–3.12; P<0.001), high TPV (≥100 mL) (OR, 8.37; 95% CI, 5.56–12.60; P<0.001), and a high BOO Index (BOOI) (≥40) (OR, 2.39; 95% CI, 1.83–3.12; P<0.001). Multivariable regression showed that AUR, the number of P-Bx, TPV, and BOOI showed statistically significant positive correlations, while 5-ARI medication history, IPSS, and BMI showed negative correlations with preoperative PSA at a 5% significance level (Supplementary Table 2).
There is still no clear consensus on how to manage LUTS/BPH patients with persistently high or increasing PSA levels with negative P-Bx results. As a result, the management of such patients may differ widely among clinicians. Urologists who specialize in voiding dysfunction tend to focus on treatment for LUTS in a timely manner. However, if these patients are managed by urological oncologists, there often may be more emphasis on PSA follow-up for potential cancer detection. These trends are more evident in tertiary hospital settings in which subspecialties in urology are clearly defined. This can cause treatment delay and unintentional negative impacts on health outcomes for patients with LUTS/BPH and persistently elevated PSA [17].
Studies have sought to understand the clinical utility of PSA cutoff values for PCa screening. Leal et al. [18] investigated the sensitivity of PSA cutoff levels of 3.0 ng/mL and 4.0 ng/mL, respectively. However, a proper understanding of the sensitivity of screening and clinical PCa detection rates is difficult. Some studies have used a PSA level of 4 ng/mL as the cutoff for the PCa work-up, while other studies have proposed lower values to reduce the risk of missing PCa [19]. We used 3.0 ng/mL as a PSA cutoff value at our institution. Our study showed a low incidental PCa rate, suggesting that this could be an effective cutoff. A Korean study analyzed the lifestyle factors associated with PSA changes using 3.0 ng/mL as a cutoff, and further studies could improve our understanding [20].
Postoperative PSA changes in BPH patients have also been reported, but mainly in TURP studies, and HoLEP studies remain few in comparison. The findings in our study are aligned with previous studies in that elevated PSA levels showed correlations with AUR, BOO, and prostate volume [11]. Patients with larger prostates have been reported in the literature to show higher PSA levels before HoLEP and had a significantly greater decrease after surgery [21]. However, a disadvantage of that paper is that the study did not focus on PSA and was not conducted under a single, strictly controlled protocol at a single institution. There is still little information on PSA changes after HoLEP.
Helfand et al. [22] followed up BPH patients postoperatively to identify predictive factors that could distinguish BPH and PCa patients. In that study, BPH patients who underwent holmium laser resection of the prostate (HoLRP) showed a postoperative decrease of 31.3%. The baseline PSA decreased from 3.2 ±1.8 ng/mL to 2.2 ±1.8 ng/mL after HoLRP. In the same study, patients who underwent TURP and were diagnosed with incidental PCa were analyzed, and their PSA levels decreased from 4.6±2.0 ng/mL to 2.4±2.2 ng/mL postoperatively. The PSA decreased in our study seemed to be higher than in conventional studies, suggestive of BPH. This could be due to the fact that the removed tissue volume in HoLEP is larger than that in TURP or HoLRP.
Ozden et al. [23] followed up patients who had preoperatively elevated PSA levels 6 months after BPH surgery. According to the study, if pretreatment P-Bx are negative and operative specimens are benign, these patients can be followed up in the same way as usual BPH patients. We also considered that a 6-month follow-up was sufficient for patients with normalized PSA, and we were able to confirm symptom improvement in most patients. If the PSA was persistently elevated or rising, a thorough evaluation and work-up were provided for PCa screening. In the future, more sophisticated PSA monitoring may improve the identification of patients requiring further therapy [24]. Further long-term analysis may be helpful to better interpret follow-up changes in PSA.
Ahyai et al. [25] reported amelioration of LUTS symptoms post-HoLEP, with patients showing improvement in the IPSS and stable recovery of QoL 3 months postoperatively. Lee et al. [26] reported that 91.8% of patients were satisfied with the surgical outcomes of HoLEP, and 94% reported that they would choose surgery if they had to reconsider. Our study showed improvements in objective and subjective symptoms in the 6-month analysis, which is consistent with recent studies [27].
It has been reported that higher baseline IPSS scores might be associated with significant symptom score improvement, but lower QoL improvement. This is due to the possible aggravation of symptoms over time, and suggests the importance of earlier treatment for patients with LUTS. The prominent improvement in IPSS scores and QoL in high-PSA patients could support the need for timely surgery for patients with elevated PSA. Considering that the high-PSA group in our study had higher baseline IPSS scores, larger prostate volume, a higher number of AUR episodes, and a higher number of P-Bx, they would likely benefit more from earlier surgical treatment.
Park et al. [28] reported that repeated biopsies were not associated with a higher PCa diagnosis rate for younger BPH patients and suggested that the risks and benefits should be carefully considered. Van Renterghem et al. [5] found that in patients with LUTS and elevated PSA, one well-performed extended P-Bx should be sufficient. In our study, the number of P-Bx did not show a statistically significant correlation with PCa, and this was consistent between the low- and high-PSA groups. This supports that additional P-Bx may not add improved diagnostic value for PCa. Therefore, in patients presenting with severe LUTS and elevated PSA, HoLEP for LUTS/BPH relief should not be unnecessarily delayed due to PSA monitoring and a PCa work-up.
Recent studies have reported that the overall incidental PCa rate after HoLEP ranged from 5.6% to 23.3% [29,30]. The incidental PCa rate in our study after surgery was low (6.3%) compared to other studies. The rigorous screening protocol used at our institution seems sufficient for cancer detection and surgical treatment decision-making for LUTS/BPH patients. Zackrisson et al. [31] reported that in patients with elevated PSA and one negative P-Bx, patients with larger prostates usually had BPH and not PCa. Capogrosso et al. [32] reported that the increased rate of low-risk incidental PCa after BPH surgery shows that clinical practice changes in PCa screening have reduced unnecessary P-Bx prior to the surgical treatment of BPH. Several strategies have been proposed in this regard, although they have not safely eliminated the need for P-Bx [33].
Magnetic resonance imaging (MRI) fusion biopsies are becoming common in countries such as the United States, though such biopsies have only recently become reimbursed [34]. Our data was collected before MRI fusion techniques were commonly used. However, our preliminary analysis showed that the cancer detection incidence after utilization of MRI screening did not significantly decrease compared to that before the utilization of MRI screening (P>0.05). Porreca et al. [35] reported that although the PCa detection rate was lower in patients with negative preoperative MRI screening results, the PCa specimen Gleason score did not show a statistically significant difference.
This study has distinct strengths compared to previous studies. First of all, this large-population long-term study analyzed more than 1,000 patients for about 10 years. Second, the patients were followed up using a prospectively designed patient study protocol with a predefined order set. Third, most of the patients in this study were referred from outside urologists, seeking optimal surgical therapy. Therefore, these patients could be considered representative of those observed in real-world clinical practice.
However, this study does have some limitations. First, we could not analyze patients who did not receive surgical treatment and compare them with those who underwent surgery. In addition, the postoperative follow-up duration was not long, because if no abnormalities were revealed, follow-up was discontinued at 6 months. There were also some patients who could not receive P-Bx prior to prostatectomy due to comorbidities, so their PSA elevation may not have been properly evaluated. Patients who had elevated PSA levels and who refused to undergo surgery were also not analyzed. Finally, we compared the incidence of PCa between two groups divided according to the baseline PSA level. However, we had no information about peripheral zone cancer because only prostatic adenoma could be removed with HoLEP. If we could analyze and compare patients with elevated PSA who do not undergo surgery, and monitor patients for longer postoperatively, we may be able to gather more information that could be valuable for clinical practice.
In patients presenting with both LUTS/BPH and concomitantly elevated PSA, timely HoLEP surgery relieved LUTS and did not show an association with higher PCa incidence. Thus, surgical treatment should not be unnecessarily delayed due to PSA monitoring for PCa in patients with BPH.
SUPPLEMENTARY MATERIALS
Supplementary Tables 1 and 2 can be found via https://doi.org/10.5213/inj.2244176.088.
Supplement Table 2.
Characteristics associated with PSA using univariable and multivariable regression analysis
NOTES
REFERENCES
1. Roehrborn CG. The utility of serum prostatic-specific antigen in the management of men with benign prostatic hyperplasia. Int J Impot Res 2008;20 Suppl 3:S19-26. PMID: 19002120



2. Nath CK, Barman B, Phukan P, Sailo SL, Dey B, Nath I, et al. Prostate-specific antigen density: a measurement to differentiate benign hypertrophy of prostate from prostate carcinoma. J Lab Physicians 2020;12:44-8. PMID: 32792793



3. Puppo P. Repeated negative prostate biopsies with persistently elevated or rising PSA: a modern urologic dilemma. Eur Urol 2007;52:639-41. PMID: 17451871


4. Katz DA, Jarrard DF, McHorney CA, Hillis SL, Wiebe DA, Fryback DG. Health perceptions in patients who undergo screening and workup for prostate cancer. Urology 2007;69:215-20. PMID: 17320653



5. van Renterghem K, Van Koeveringe G, Achten R, van Kerrebroeck P. A new algorithm in patients with elevated and/or rising prostatespecific antigen level, minor lower urinary tract symptoms, and negative multisite prostate biopsies. Int Urol Nephrol 2010;42:29-38. PMID: 19496018



6. Cho HJ, Shin SC, Cho JM, Kang JY, Yoo TK. The role of transurethral resection of the prostate for patients with an elevated prostatespecific antigen. Prostate Int 2014;2:196-202. PMID: 25599076



7. Tinmouth WW, Habib E, Kim SC, Kuo RL, Paterson RF, Terry CL, et al. Change in serum prostate specific antigen concentration after holmium laser enucleation of the prostate: a marker for completeness of adenoma resection? J Endourol 2005;19:550-4. PMID: 15989443


8. Elkhodair S, Parmar HV, Vanwaeyenbergh J. The role of the IPSS (International Prostate Symptoms Score) in predicting acute retention of urine in patients undergoing major joint arthroplasty. Surgeon 2005;3:63-5. PMID: 15861938


9. Kang M, Kim M, Choo MS, Paick JS, Oh SJ. Urodynamic features and significant predictors of bladder outlet obstruction in patients with lower urinary tract symptoms/benign prostatic hyperplasia and small prostate volume. Urology 2016;89:96-102. PMID: 26683755


10. Hwang SI, Lee HJ. The future perspectives in transrectal prostate ultrasound guided biopsy. Prostate Int 2014;2:153-60. PMID: 25599070



11. Xu L, Hu X, Zhu Y, Lu J, Xu Y, Wang G. Additional value of the ratio of serum total testosterone to total prostate-specific antigen in the diagnosis of prostate cancer in a Chinese population. Andrologia 2018 Mar;50(2):https://doi.org/10.1111/and.12872. [Epub]. PMID: 28752596


12. Kim SJ, Lee J, An DH, Park CH, Lim JH, Kim HG, et al. A randomized controlled comparison between periprostatic nerve block and pelvic plexus block at the base and apex of 14-core prostate biopsies. World J Urol 2019;37:2663-9. PMID: 30864006



13. Yoo S, Son H, Oh S, Park J, Cho SY, Cho MC, et al. A novel biopsyrelated parameter derived from location and relationship of positive cores on standard 12-core trans-rectal ultrasound-guided prostate biopsy: a useful parameter for predicting tumor volume compared to number of positive cores. J Cancer Res Clin Oncol 2018;144:135-43. PMID: 28939976



14. Oh SJ. Current surgical techniques of enucleation in holmium laser enucleation of the prostate. Investig Clin Urol 2019;60:333-42. PMID: 31501795




15. Sarıkaya S, Resorlu M, Oguz U, Yordam M, Bozkurt OF, Unsal A. Evaluation of the pathologic results of prostate biopsies in terms of age, Gleason score and PSA level: our experience and review of the literature. Arch Ital Urol Androl 2014;86:288-90. PMID: 25641453



16. Piao S, Choo MS, Kim M, Jeon HJ, Oh SJ. Holmium laser enucleation of the prostate is safe for patients above 80 years: a prospective study. Int Neurourol J 2016;20:143-50. PMID: 27377947




17. Ilic D, Djulbegovic M, Jung JH, Hwang EC, Zhou Q, Cleves A, et al. Prostate cancer screening with prostate-specific antigen (PSA) test: a systematic review and meta-analysis. BMJ 2018;362:k3519. PMID: 30185521



18. Leal J, Welton NJ, Martin RM, Donovan J, Hamdy F, Neal D, et al. Estimating the sensitivity of a prostate cancer screening programme for different PSA cut-off levels: a UK case study. Cancer Epidemiol 2018;52:99-105. PMID: 29278842


19. Rashid MM, Alam AK, Habib AK, Rahman H, Hossain AK, Salam MA, et al. Efficacy of lower cut off value of serum prostate specific antigen in diagnosis of prostate cancer. Bangladesh Med Res Counc Bull 2012;38:90-3. PMID: 23540183



20. Woo HY, Park H, Kwon MJ, Chang Y, Ryu S. Association of prostate specific antigen concentration with lifestyle characteristics in Korean men. Asian Pac J Cancer Prev 2012;13:5695-9. PMID: 23317241


21. Romero-Otero J, García-Gómez B, García-González L, García-Rojo E, Abad-López P, Justo-Quintas J, et al. Critical analysis of a multicentric experience with holmium laser enucleation of the prostate for benign prostatic hyperplasia: outcomes and complications of 10 years of routine clinical practice. BJU Int 2020;126:177-82. PMID: 32020749



22. Helfand BT, Anderson CB, Fought A, Kim DY, Vyas A, McVary KT. Postoperative PSA and PSA velocity identify presence of prostate cancer after various surgical interventions for benign prostatic hyperplasia. Urology 2009;74:177-83. PMID: 19428074


23. Ozden C, Inal G, Adsan O, Yazici S, Ozturk B, Cetinkaya M. Detection of prostate cancer and changes in prostate-specific antigen (PSA) six months after surgery for benign prostatic hyperplasia in patients with elevated PSA. Urol Int 2003;71:150-3. PMID: 12890951



24. Nunez R, Hurd KJ, Noble BN, Castle EP, Andrews PE, Humphreys MR. Incidental prostate cancer revisited: early outcomes after holmium laser enucleation of the prostate. Int J Urol 2011;18:543-7. PMID: 21592233


25. Ahyai SA, Marik I, Ludwig TA, Becker A, Asdjodi S, Kluth L, et al. Super early detailed assessment of lower urinary tract symptoms after holmium laser enucleation of the prostate (HoLEP): a prospective study. World J Urol 2020;38:3207-17. PMID: 32086571



26. Lee YJ, Oh SA, Kim SH, Oh SJ. Patient satisfaction after holmium laser enucleation of the prostate (HoLEP): a prospective cohort study. PLoS One 2017;12:e0182230. PMID: 28793314



27. Cho MC, Kim JK, Ha SB, Ku JH, Paick JS. Self-assessed goal achievement (SAGA) after Holmium laser enucleation of the prostate (HoLEP): association with patients’ postoperative satisfaction. PLoS One 2018;13:e0203825. PMID: 30212587



28. Park HG, Ko OS, Kim YG, Park JK. Efficacy of repeated transrectal prostate biopsy in men younger than 50 tears with an elevated prostate-specific antigen concentration (>3.0 ng/mL): risks and benefits based on biopsy results and follow-up status. Korean J Urol 2014;55:249-53. PMID: 24741413



29. Elkoushy MA, Elshal AM, Elhilali MM. Incidental prostate cancer diagnosis during holmium laser enucleation: assessment of predictors, survival, and disease progression. Urology 2015;86:552-7. PMID: 26216838


30. Rosenhammer B, Lausenmeyer EM, Mayr R, Burger M, Eichelberg C. HoLEP provides a higher prostate cancer detection rate compared to bipolar TURP: a matched-pair analysis. World J Urol 2018;36:2035-41. PMID: 29858700



31. Zackrisson B, Aus G, Lilja H, Lodding P, Pihl CG, Hugosson J. Follow-up of men with elevated prostate-specific antigen and one set of benign biopsies at prostate cancer screening. Eur Urol 2003;43:327-32. PMID: 12667711


32. Capogrosso P, Capitanio U, Vertosick EA, Ventimiglia E, Chierigo F, Oreggia D, et al. Temporal trend in incidental prostate cancer detection at surgery for benign prostatic hyperplasia. Urology 2018;122:152-7. PMID: 30138683



33. Borer JG, Sherman J, Solomon MC, Plawker MW, Macchia RJ. Age specific prostate specific antigen reference ranges: population specific. J Urol 1998;159:444-8. PMID: 9649260


34. Ryoo H, Kang MY, Sung HH, Chang Jeong B, Seo SI, Jeon SS, et al. Detection of prostate cancer using prostate imaging reporting and data system score and prostate-specific antigen density in biopsynaive and prior biopsy-negative patients. Prostate Int 2020;8:125-9. PMID: 33102394



35. Porreca A, Giampaoli M, Bianchi L, D’Agostino D, Romagnoli D, Bianchi FM, et al. Preoperative multiparametric prostate magnetic resonance imaging: a safe clinical practice to reduce incidental prostate cancer in Holmium laser enucleation of the prostate. Cent European J Urol 2019;72:106-12. PMID: 31482016


Fig. 1.
Changes in prostate-specific antigen (PSA) level between baseline and postoperative 6 months in both low and high PSA groups. (A) Changes in PSA from baseline low and high PSA groups at postoperative 6 months; the red lines indicate the patients in which PSA increased postoperatively. (B) Diagram of patient re-grouping at postoperative sixth months according to the PSA levels in both baseline high and low PSA groups; the orange- colored sections indicate percentage of patient with high PSA levels.
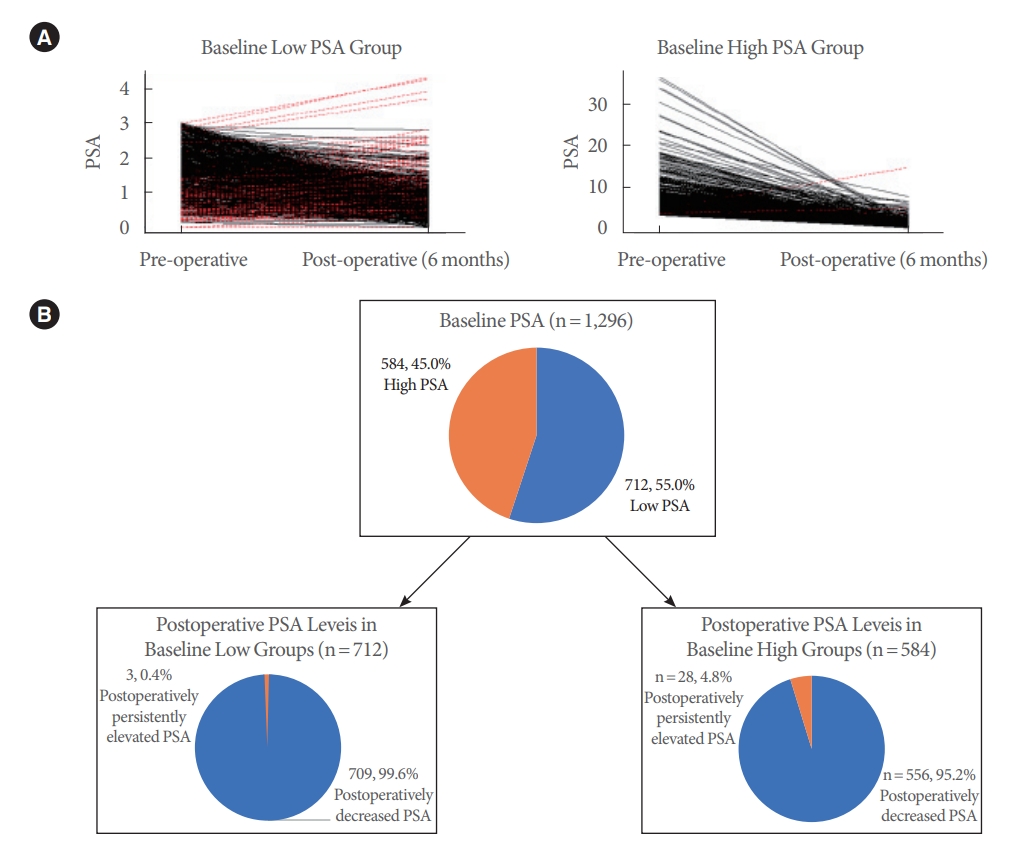
Table 1.
Baseline patient characteristics
| Characteristic | Whole population (n = 1,296) | Low-PSA group PSA < 3.0 ng/mL (n = 712) | High-PSA group PSA≥ 3.0 ng/mL (n=584) | P-valuea) | |
|---|---|---|---|---|---|
| Age (yr) | 69.7 ± 6.8 | 69.5 ± 6.6 | 69.8 ± 7.2 | 0.55 | |
| Body mass Index (kg/m2) | 24.2 ± 2.9 | 24.5 ± 3.0 | 24.0 ± 2.8 | 0.14 | |
| Symptom duration (mo) | 42.5 ± 64.6 | 43.9 ± 72.9 | 40.7 ± 52.8 | 0.37 | |
| Preoperative medications for LUTS | |||||
| Alpha blockers | 923 (71.2) | 508 (71.3) | 415 (71.0) | 0.23 | |
| 5-α reductase inhibitors | 447 (34.5) | 253 (35.5) | 194 (33.2) | 0.17 | |
| IPSS-storage symptom score | 8.0 ± 4.6 | 7.9 ± 4.5 | 8.1 ± 4.6 | 0.55 | |
| IPSS-obstructive symptom score | 11.8 ± 6.7 | 12.1 ± 6.4 | 11.5 ± 6.9 | 0.20 | |
| IPSS-total score | 19.8 ± 10.5 | 20.0 ± 10.2 | 19.6 ± 10.8 | 0.41 | |
| IPSS, quality of life score | 4.2 ± 1.5 | 4.2 ± 1.5 | 4.3 ± 1.5 | 0.42 | |
| Maximum flow rate (mL/sec) | 9.3 ± 4.7 | 9.6 ± 4.9 | 9.2 ± 4.5 | 0.13 | |
| Postvoided residual volume (mL) | 130.5 ± 169.0 | 133.4 ± 139.0 | 129.6 ± 170.0 | 0.38 | |
| Acute urinary retention (times) | < 0.001 | ||||
| 0 | 1,002 (77.3) | 599 (84.2) | 403 (69.0) | ||
| 1 | 261 (20.1) | 103 (14.4) | 158 (27.1) | ||
| 2 | 21 (1.6) | 6 (0.8) | 15 (2.6) | ||
| 3 | 9 (0.7) | 2 (0.3) | 7 (1.2) | ||
| 4 | 3 (0.2) | 2 (0.3) | 1 (0.2) | ||
| Prostate-specific antigen (ng/mL) | 4.0 ± 4.1 | 1.6 ± 0.8 | 6.9 ± 4.7 | < 0.001 | |
| Prostate volume | |||||
| Total prostate volume (mL) | 68.3 ± 33.6 | 54.3 ± 23.3 | 85.4 ± 36.3 | < 0.001 | |
| Transitional zone volume (mL) | 39.6 ± 26.6 | 29.0 ± 18.8 | 52.3 ± 28.8 | < 0.001 | |
| Prostate biopsy (times) | < 0.001 | ||||
| 0 | 854 (65.9) | 634 (89.1) | 220 (37.7) | ||
| 1 | 357 (27.5) | 70 (9.8) | 287 (49.1) | ||
| 2 | 57 (4.4) | 6 (0.8) | 51 (8.7) | ||
| 3 | 21 (1.6) | 1 (0.1) | 20 (3.4) | ||
| 4 | 6 (0.5) | 1 (0.1) | 5 (0.9) | ||
| 5 | 1 (0.1) | 0 (0) | 1 (0.2) | ||
| Bladder outlet obstruction index | 44.5 ± 2.9 | 39.0 ± 25.0 | 51.1 ± 32.0 | < 0.001 | |
| Maximal detrusor pressure at Qmax | 67.1 ± 27.5 | 60.8 ± 25.2 | 74.9 ± 28.1 | 0.002 | |
Table 2.
Comparison of functional outcomes between low and high-PSA groups
| Variable | Whole population (n=1,296) | Low-PSA group PSA< 3.0 ng/mL (n=712) | High-PSA group PSA≥ 3.0 ng/mL (n=584) | P-valuea) | |
|---|---|---|---|---|---|
| Baseline | |||||
| IPSS-storage symptom score | 8.0 ± 4.6 | 7.9 ± 4.5 | 8.1 ± 4.6 | 0.55 | |
| IPSS-obstructive symptom score | 11.8 ± 6.7 | 12.1 ± 6.4 | 11.5 ± 6.9 | 0.20 | |
| IPSS-total score | 19.8 ± 10.5 | 20.0 ± 10.2 | 19.6 ± 10.8 | 0.41 | |
| IPSS, QoL score | 4.2 ± 1.5 | 4.2 ± 1.5 | 4.3 ± 1.5 | 0.42 | |
| Maximum flow rate (mL/sec) | 9.3 ± 4.7 | 9.6 ± 4.9 | 9.2 ± 4.5 | 0.13 | |
| Postvoid residual volume (mL) | 130.5 ± 169.0 | 133.4 ± 139.0 | 129.6 ± 170.0 | 0.38 | |
| Short-term postoperative outcomes (3 mo) | |||||
| IPSS-storage symptom score | 5.6 ± 4.9 | 5.8 ± 5.1 | 5.3 ± 4.8 | 0.06 | |
| IPSS-obstructive symptom score | 3.7 ± 6.8 | 4.4 ± 7.3 | 2.9 ± 6.1 | 0.001 | |
| IPSS-total score | 8.6 ± 11.0 | 9.5 ± 11.7 | 7.5 ± 10.0 | 0.001 | |
| IPSS, QoL score | 1.9 ± 1.9 | 2.0 ± 2.1 | 1.7 ± 1.7 | 0.003 | |
| Maximum flow rate (mL/sec) | 21.5 ± 10.9 | 21.0 ± 12.2 | 22.0 ± 14.8 | 0.02 | |
| Postvoid residual volume (mL) | 88.5 ± 93.5 | 89.6 ± 102.9 | 87.2 ± 67.8 | 0.69 | |
| Δ IPSS-storage symptom score | -2.4 ± 5.2b) | -2.1 ± 5.1b) | -2.8 ± 5.3b) | 0.26 | |
| Δ IPSS-obstructive symptom score | -8.0 ± 7.8b) | -8.2 ± 7.4b) | -7.7 ± 7.8b) | 0.21 | |
| Δ IPSS-total score | -11.2 ± 11.9b) | -10.9 ± 11.7b) | -12.0 ± 12.2b) | 0.19 | |
| Δ IPSS, QoL score | -2.3 ± 2.6b) | -2.2 ± 2.5b) | -2.9 ± 2.5b) | 0.41 | |
| Δ Maximum flow rate (mL/sec) | 12.2 ± 6.1b) | 11.0 ± 7.3b) | 13.4 ± 39.9b) | 0.23 | |
| Δ Postvoid residual volume (mL) | -41.9 ± 99.8b) | -43.8 ± 100.2b) | -42.0 ± 99.8b) | 0.43 | |
| Midterm postoperative outcomes (6 mo) | |||||
| IPSS-storage symptom score | 4.4 ± 4.9 | 4.5 ± 4.6 | 4.1 ± 5.1 | 0.15 | |
| IPSS-obstructive symptom score | 3.6 ± 6.8 | 4.1 ± 6.7 | 3.0 ± 7.0 | 0.01 | |
| IPSS-total score | 7.5 ± 11.1 | 8.2 ± 10.8 | 6.7 ± 11.5 | 0.01 | |
| IPSS, QoL score | 1.6 ± 1.9 | 1.8 ± 1.8 | 1.4 ± 1.9 | 0.001 | |
| Maximum flow rate (mL/sec) | 22.0 ± 13.2 | 20.7 ± 12.0 | 23.5 ± 11.3 | 0.001 | |
| Postvoid residual volume (mL) | 64.2 ± 75.1 | 61.4 ± 84.6 | 67.8 ± 61.1 | 0.22 | |
| Δ IPSS-storage symptom score | -3.6 ± 5.1c) | -3.9 ± 4.8c) | -3.3 ± 5.4c) | 0.06 | |
| Δ IPSS-obstructive symptom score | -8.2 ± 7.5c) | -8.6 ± 7.1c) | -7.8 ± 8.0c) | 0.08 | |
| Δ IPSS-total score | -12.3 ± 11.9c) | -12.4 ± 11.1c) | -11.1 ± 12.7c) | 0.06 | |
| Δ IPSS, QoL score | -2.6 ± 2.7c) | -2.5 ± 2.6c) | -2.5 ± 2.7c) | 0.42 | |
| Δ Maximum flow rate (mL/sec) | 12.7 ± 5.9c) | 12.1 ± 8.2c) | 13.3 ± 7.6c) | 0.56 | |
| Δ Postvoid residual volume (mL) | -65.3 ± 102.1c) | -62.1 ± 110.2c) | 61.9 ± 100.2c) | 0.34 | |
Table 3.
Operative pathology according to baseline PSA groups
| Variable | Whole population (n=1,296) | Low-PSA group PSA< 3.0 ng/mL (n=712) | High-PSA group PSA≥ 3.0 ng/mL (n=584) | P-valuea) | |
|---|---|---|---|---|---|
| Operative pathology | 0.21 | ||||
| Nodular hyperplasia | 1,214 (93.7) | 671 (94.2) | 543 (93.0) | ||
| Adenocarcinoma | 82 (6.3) | 41 (5.8) | 41 (7.0) | ||
| Gleason score | 0.21 | ||||
| 5 (2+3) | 1 (1.2) | 1 (2.4) | 0 (0) | ||
| 6 (3+3) | 72 (87.8) | 33 (80.5) | 39 (95.1) | ||
| 7 (3+4) | 8 (9.8) | 6 (14.7) | 2 (4.9) | ||
| 8 (4+4) | 1 (1.2) | 1 (2.4) | 0 (0) | ||
| Tumor percentage | 0.33 | ||||
| 1 | 47 (57.4) | 21 (51.3) | 26 (63.4) | ||
| 2 | 18 (21.9) | 11 (26.9) | 7 (17.1) | ||
| 3 | 10 (12.3) | 4 (9.7) | 6 (14.6) | ||
| 5 | 2 (2.4) | 2 (4.9) | 0 (0) | ||
| 6 | 1 (1.2) | 1 (2.4) | 0 (0) | ||
| 7 | 1 (1.2) | 1 (2.4) | 0 (0) | ||
| 10 | 2 (2.4) | 0 (0) | 2 (4.9) | ||
| 20 | 1 (1.2) | 1 (2.4) | 0 (0) | ||





