Clinical Approach to Recurrent Voiding Dysfunction, Dysuria, and Pelvic Pain Persisting for at Least 3 Months
Article information
Abstract
There are several patients with urination problems and urethral and pelvic discomfort. Usually, these patients’ symptoms are persistent and ambiguous; therefore, it is difficult to find underlying diseases associated with the patient’s symptoms. In addition, there are various conditions such as overactive bladder, cystitis, and interstitial cystitis/bladder pain syndrome (IC/BPS). Sometimes patients with other chronic disorders such as fibromyalgia, inflammatory bowel syndrome, and vulvodynia show urination problems and pelvic pain. Thus, a patient-centered approach is important to find the cause of chronic urination problems and pelvic pain. Moreover, IC/BPS should be considered during the diagnostic process because the clinical characteristics of IC/BPS are diverse. In this narrative review, we suggest an integral approach for the diagnosis and treatment of IC/ BPS.
AWARENESS ABOUT INTERSTITIAL CYSTITIS/BLADDER PAIN SYNDROME
Women comprise more than half of the world’s population. Voiding dysfunction and reproductive disorders can occur in both men and women. However, many people still perceive urology as an area of medicine primarily for men. Hence, women of nearly all ages, especially those suffering from recurrent voiding dysfunction, dysuria, and pelvic pain, commonly miss opportunities for proper diagnosis and treatment. The disease typically behind these symptoms is termed chronic cystitis, bladder pain syndrome, interstitial cystitis, or interstitial cystitis/bladder pain syndrome (IC/BPS). It is not caused by bacterial infection. Patients report abnormal voiding patterns such as urinary frequency, urinary urgency, and nocturia, as well as pain in pelvic regions, including the lower abdomen, flanks, and genital area. The disease can occur in men and women of any age, but it is seen more frequently after sexual maturity, especially among middle-aged women over 40 years old [1-3].
No clear cause of IC/BPS has been discovered, and its subjective symptoms resemble those of acute cystitis and vaginitis caused by bacterial infection. For this reason, it is often mistaken for simple urogynecological diseases, leading to unnecessary antibiotic exposure. Many patients are finally diagnosed with IC/BPS after a considerable amount of time, by which point they have been infected with antibiotic-resistant bacteria. If a patient reports recurrent pain and abnormal sensations in the pelvis, lower abdomen, flanks, urethra, vagina, and/or clitoris, along with voiding symptoms such as urinary frequency, urinary urgency, and nocturia, clinicians must investigate whether these symptoms have persisted for at least 3 months and whether the patient felt anxiety due to the pain and abnormal sensations. Once patients receive treatment, the symptoms may disappear initially but later reappear. As the disease progresses beyond the initial stage, the disappearance of symptoms is delayed and symptom relief may no longer be observed. Since the exact cause is unknown, patients can eventually experience chronic and severe anxiety and depression. Therefore, we can get hints by listening closely to the patients’ descriptions of discomfort that comes to me is like a well-written essay. For accurate diagnosis and targeted therapy, we advise both clinicians and patients to understand the disease progression from the onset of symptoms to the present as an inevitable process from the perspectives of patients and their clinicians. Then, they should consider various viewpoints and endeavor to understand them. Moreover, from the patient’s perspective, there is no concept of “disease progression.” Clinicians should therefore empathize with patients, who perceive the disease to have suddenly appeared at the moment when they experience symptoms. Diagnose IC/BPS, both clinicians and patients should begin with awareness and an investigative mindset. Although some medical staff still consider those diseases very rare and potentially unfamiliar, we assert that they affect a substantial number of patients. We shared my experiences: more than 13 million people are suffering from pain syndrome even in the United States, but quite a few patients have been neglected for years or have been misdiagnosed. The disease may even be mistaken for colon disease, heart disease, or pelvic or lower extremity skeletal disease. It is now necessary to raise awareness of IC and BPS through proactive communication between medical consumers and providers based on accurate information [4-9].
If a patient has been treated for cystitis and urinary tract infection and a urine bacterial culture is negative, IC/BPS should be considered. The disease should also be suspected if the pain is limited to the lower abdomen, flanks, waist, urethra, vagina, clitoris, and/or perineal region; if pain exacerbation and relief are linked to the menstrual cycle; and if the pain is related to certain activities such as sexual intercourse or ejaculation. In particular, the bladder should be suspected as a source of symptoms if the patient has pain in the lower extremities (such as on one side of the buttock and thigh), the pain is intermittently or continuously related to bladder filling, and no orthopedic abnormality exists. In these cases, the disease may have progressed significantly. If the pains described above are intermittent or continuous and accompanied by lower urinary tract symptoms such as urinary frequency, urinary urgency, and stress and urge incontinence, patients should ask themselves why they often go to the bathroom—because of an urge to urinate, sickness, or anxiety about getting sick. While long thought to appear only in women, BPS and IC are also found in men. Unlike in women, in men the pain appears in the testicles, scrotum, anus, and perineum, as well as the lower abdomen, urethra, lumbar spine, and tailbone, regardless of bladder filling. Typically, the prostate is first identified as the causative target, and the condition is sometimes simply diagnosed as benign prostatic hyperplasia and prostatitis. At this point, the bladder should be considered as a causative target [10-15].
CONSIDERING POINTS FOR THE DIAGNOSIS OF INTERSTITIAL CYSTITIS/BLADDER PAIN SYNDROME
Patients have many options for treatment of IC/BPS. Depending on the patient’s condition and age, various treatments are possible, including lifestyle correction and observational treatment, active exercise therapy involving repeated muscle relaxation and contraction, passive exercise therapy, oral drug therapy, intravesical injection therapy, urethral-vaginal injection therapy, and surgical treatment. Although the disease recurs frequently, and the treatment process is exhausting to patients and medical staff, treatment also help the patients. Therefore, an accurate diagnosis and proper professional treatment can contribute to society by improving patients’ overall quality of life and their routine daily lives. The pains and symptoms previously mentioned are the main diagnostic criteria for this group of diseases, and urinary frequency is also commonly observed at the initial stage. Pelvic pain due to bladder filling is a common symptom, but is not currently essential for diagnosis. Among lower urinary tract symptoms, patients commonly misinterpret urinary urgency as pain or fear of urinating unintentionally, but this is also not a requirement for diagnosis. Again, current bladder-related pain and discomfort are the primary and essential diagnostic requirements [16-25].
Mast cells involved in allergic reactions are distributed throughout the blood vessels, lymph glands, and nerves of the bladder, as well as the bladder smooth muscle and mucosa. Mast cells activated by the involvement of neurological, immune, and endocrine factors are thought to play a central role in the progression of IC/BPS in many patients [26].
One-fourth of patients who meet the international diagnostic criteria for IC/BPS show a negative result on a potassium chloride sensitivity test, whereas the test result is positive in most women with radiation cystitis, urinary tract infection, chronic prostatitis, and pelvic pain. Thus, this test shows low sensitivity and specificity in diagnosis and is not helpful for therapeutic decision-making.
In fewer than 30% of patients with symptoms of IC/BPS, bladder ulcers, formerly called Hunner ulcers, are found. Notably, the frequency of detecting ulcers varies depending on the diagnostic method. Bladder ulcers are easily identified in general outpatient-based cystoscopy, but some clinicians say that bladder hydrodistension is an easier method to identify vulnerable ulcers. I tend to agree with the latter point of view and use bladder hydrodistension in practice [27-29].
The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) has developed diagnostic criteria for BPS. If these criteria are strictly applied, the sensitivity of the diagnosis decreases, while the specificity increases. Of note, 90% of the clinical experts who set the NIDDK diagnostic criteria agree with the diagnosis according to these criteria. However, in practice, 60% of patients clinically diagnosed with IC/BPS were shown to not satisfy the NIDDK diagnostic criteria. Thus, using these criteria probably excludes many patients with symptom clusters that are reasonable for diagnosis. That is, the NIDDK criteria are subject to the weakness of high specificity but low sensitivity. IC/BPS is often accompanied by diabetes, fibromyalgia, irritable bowel syndrome (IBS), chronic fatigue syndrome, or atopic allergic reaction. Currently, researchers at the NIDDK’s Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network are investigating the potential associations between disease groups [30-35]. If a patient has a history of using antipsychotic drugs (e.g., ketamine cystitis shows similar symptoms to those of IC/BPS), it is common for inflammation of the lower and upper urinary tract to be concomitantly present. Therefore, if inflammation of the lower urinary tract not due to bladder cancer or tuberculous cystitis is identified on endoscopy in female patients, it is reasonable to focus on IC/BPS treatment, although another diagnostic therapeutic approach is appropriate if the clinician is unaware of the patient’s drug history [36-39].
When multiple conservative treatments fail, bladder chemodenervation therapy involving the off-label use of botulinum toxin A can be considered. This approach is possible when obstructive voiding symptoms are accompanied by hypertonic pelvic floor muscle abnormalities, along with pelvic floor muscle hypertonia and obvious muscle tenderness [40-43]. However, it should be used with caution in patients with obstructive voiding difficulty.
Currently, the characteristic histological findings of IC/BPS serve no known diagnostic or prognostic value. Even severe microscopic abnormalities are known not to indicate a poor prognosis. Various opinions exist about the meaning of histopathological detection and findings in IC/BPS patients. Currently, 2 opinions are widely held: (1) these findings exclude other diseases showing symptoms similar to those mentioned above and (2) these findings are meaningful as a way to identify inflammatory diseases with similar symptoms. Hence, it is not reasonable to make a diagnostic, therapeutic, and prognostic plan based only on histological findings.
Currently, IC/BPS is considered a genitourinary and anal pain syndrome. However, vulvodynia, testicular pain, perineal pain, genital pain, and anal pain are well known and described as symptomatic syndromes, but the mechanisms and treatments specific to the causes of each pain are not well known. Below are examples of the disease group of prostatitis, which is common in men. Bacterial prostatitis is a disease entity for which diagnostic, therapeutic, and prognostic plans—including its causes and responses to treatment of the causative organism —are well known. In other words, National Institutes of Health (NIH) category 1 and NIH category 2 are included in this disease group and are not correlated to IC/BPS, unlike NIH category 3 (chronic pelvic pain syndrome [CPPS]/nonbacterial prostatitis). The abovementioned explanation will be clearer when looking at each group of diagnoses of exclusion in the form of a cluster of reported symptoms. Among lower urinary tract symptoms, when pelvic pain accompanies storage symptoms, the most common diseases in the differential diagnosis are bladder outlet obstruction, NIH type prostatitis, BPS, and rarely bacterial cystitis and bladder carcinoma in situ. In medical practice, many patients experience unnecessary prostate and bladder neck surgery due to errors in the differential diagnosis when patients are analyzed based on symptoms [44-47]. Therefore, the importance of the differential diagnosis must not be overlooked.
The relationship with urodynamic studies (UDS) can be examined in 2 representative and common aspects. Cystometry in IC/BPS patients generally shows normal findings, and the characteristic findings are a decrease in bladder capacity and hypersensitivity. In particular, repeated pain accompanying bladder filling strongly suggests IC. Although the mechanism of low bladder compliance is not well known, it may be confused with urge urinary incontinence (a common disease in which bladder compliance decreases) and uninhibited detrusor contraction (a finding of cystometry in frequency-urgency syndrome and neurogenic bladder) [48]. To summarize, bladder compliance is normal up to the point of bladder filling where pain occurs. The second aspect relates to the diagnostic and therapeutic implications when symptoms include bladder pain, no urinary urgency, and detrusor overactivity found on examination. In this case, IC/BPS is excluded diagnostically, a urinary tract infection may be considered, and accordingly, a tendency may exist to prioritize the administration of antibiotics and anticholinergics and botulinum toxin for treatment. However, clinically meaningless detrusor overactivity can be found on examination in 15% of IC/BPS patients, which is the frequency of involuntary contractions in ambulatory UDS among the general population. Hence, clinicians should be aware that there are no diagnostic or therapeutic implications for the reasons described above.
Glomerulation is not a characteristic endoscopic finding only in IC/BPS patients. Therefore, it has little diagnostic and therapeutic value. It can also appear in patients receiving treatment for radiation cystitis and bladder cancer and chemotherapy, and in patients who have not filled their bladder for a long time such as patients on dialysis or urinary diversion. Glomerulation on cystoscopy is not medically significant, particularly in asymptomatic patients [49].
A potential explanatory theory for the effective mechanism of tricyclic antidepressants (e.g., amitriptyline) as therapeutic agents explains the effects by the following mechanisms rather than the original antidepressant effect of the drug: (1) a positive effect on sleep, (2) a blocking effect of the acetylcholine receptor among autonomic nerve system receptors in the bladder, (3) a blocking effect on the histamine receptor, and (4) a palliative effect on neuropathic pain of the somatosensory system. In particular, it is effective to relieve symptoms in patients with overactive bladder, whereas caution is necessary for patients with obstructive voiding symptoms. Moreover, the drug dose for relieving symptoms in IC/BPS patients is lower than that for major depressive symptoms [50-54].
CONSIDERING POINTS FOR THE TREATMENT OF INTERSTITIAL CYSTITIS/BLADDER PAIN SYNDROME
I often ponder how to best make decisions regarding patients’ diagnoses and therapeutic prognoses. Although there have been many trials and studies such as potassium chloride testing, intravesical heparin injection, bladder epithelium biopsy, and UDS, cystoscopy and lower-pressure hydrodistension are most helpful. Generally, surgical methods of IC/BPS and a diagnostic method of anesthetic bladder hydrodistension are frequently used. One of the main purposes is to confirm the presence of a Hunner lesion, and 30%–50% of patients show short-term symptom improvements, whereas 30% of patients paradoxically show short-term symptom exacerbation. In particular, an anesthetic maximum bladder capacity of less than 200 mL can be a sign of poor therapeutic prognosis. If the abovementioned ulcer lesions are observed in patients, an excellent therapeutic effect can often be achieved by performing resection, fulguration, or steroid injection on lesion. However, no therapeutic effects have been proven for treatments suggested by some researchers such as reduction cystoplasty, sympathectomy and intraspinal alcohol injection, cystolysis, and transvesical infiltration of the pelvic plexuses with phenol [55-60].
Although several treatments including bacillus Calmette– Guérin 4% lidocaine, botulinum toxin, and heparin were tried, no treatment has been proven effective in double-blind placebo-controlled trials to date. Pentosan polysulfate sodium (PPS), a typical oral medication, is an oral analogue of heparin and targets the restoration of glycosaminoglycan (GAG) of urothelium. The currently suggested mechanism is to correct dysfunction of the GAG layer and particularly focuses on the correction of permeability abnormalities. In certain patient groups, caution is necessary since it is associated with visual impairment due to pigmentary maculopathy as a result of long-term administration [61,62].
Opioids, unlike other analgesics, do not have a dose limit for their analgesic effects. Opioids can be useful for IC/BPS patients, although attention should be paid to the fact that opioids can cause physical dependence due to chronic use as the dose is increased to the extent that a patient can endure the side effects of narcotic analgesics and it is necessary to comply with common medical precautions when using opioids.
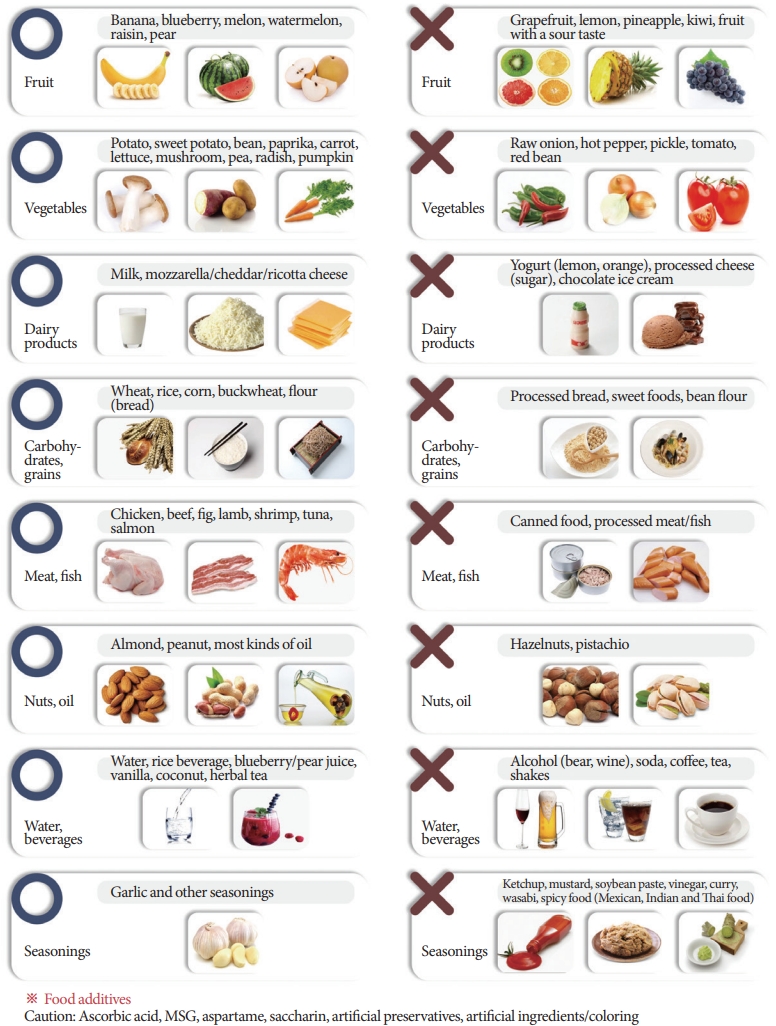
Although diet control (Figs. 1, 2), physical therapy, and oral administration of PPS can be considered as the primary initial treatment of IC/BPS, the importance of disease awareness and education should be emphasized above all. In particular, patients should understand that even if they follow a reasonable treatment plan, symptom relief can be experienced as chronic progression and repeated improvement and exacerbation of symptoms. This initial education can prevent wasteful doctor shopping, increases in medical costs due to repetitive and excessive examinations, and desperation or extreme damage due to a lack of understanding of the disease. Patients can make the best therapeutic choice in a long-term treatment process focusing on symptoms [63-65].
Below, I present some opinions about situations when invasive surgery is performed due to severely progressed IC/BPS. When a patient has severe symptoms and an accompanying Hunner lesion, anesthetic bladder hydrodistension and Hunner lesion resection are planned and performed. Although bladder hydrodistension is not commonly chosen when the anesthetic maximum bladder capacity is less than 100 mL, I, as an urologist performing surgery, would like to emphasize that clinicians should be aware of unusual prognostic issues when making surgical decisions. In particular, if urethral pain exists among IC/BPS symptoms, it should be kept in mind that clean intermittent catheterization should be maintained in over 30% of patients after bladder hydrodistension. Since Hunner lesions always exist in the supratrigonal area of the trigone, trigonal sparing and supratrigonal cystectomy with bladder augmentation should be considered primarily as surgical methods.
Regarding the risk factors for IC/BPS, the Boston Area Community Health Survey and several community studies of similar size have mentioned emotional, sexual, and physical abuse. In a study conducted in Michigan (control group, n=464; patient group, n=215), a difference in exposure to the abovementioned risk factors was observed between the control group (22%) and the patient group (37%). A particularly interesting point was that sexual abuse as a risk factor was associated with fewer cases of exacerbated voiding symptoms, but a remarkable increase in the exacerbation of painful symptoms. Although various opinions exist on the reliability of these 2 studies, it is not reasonable to subdivide the changes in symptoms caused by exposure to risk factors in the medical field. In other words, the possibility of each patient’s sensitivity and specificity to exposure to each risk factor should be considered, and clinicians should be aware of the necessity of collaboration with other departments when the differential diagnosis includes various possible diseases. It should not be recognized as a behavioral pattern associated with IC/BPS, and side effects of treatment or association with patients’ complaints particularly should not be denied.
Hunner lesions could reasonably be recognized as a distinct phenotype distinguished clearly as a separate patient group with unique therapeutic and prognostic specificity, rather than as a comorbid condition in BPS patients. Hunner lesions can be identified by endoscopic and histological examinations, and their frequency increases in elderly patients. As a point of therapeutic significance, the clinical effects of resection, fulguration, and steroid injection have been proven.
Intravesical injection therapy, as a secondary treatment, has a therapeutic effect on bladder pain, especially in bladder-centric pain, and has some effect on fibromyalgia, Hunner lesions, neuropathic pain, and small-capacity bladder. However, no special effect has been observed on vulvodynia accompanying high-tone pelvic floor dysfunction, and caution is necessary since the therapy can worsen the abovementioned symptoms.
SUMMARY
The clinical diagnosis of IC/BPS is based on pain, pressure, and discomfort associated with the bladder accompanying lower urinary tract symptoms that persists 6 weeks or more without inflammation or other identified causes. It is generally linked to bladder filling and can be defined as a disease entity for which a diagnosis of exclusion must be conducted using specific tests and markers for other diseases. The frequency of this condition is rapidly increasing as the diagnostic criteria take the form of a clinical symptom complex. Epidemiologically, 3 million women and 2 million men in the United States are known to experience IC/BPS symptoms. In the clinical field, the number of female patients is higher, with a ratio of 5 to 1. Although the accompanying sexual dysfunction in female patients has a clear negative impact on physical and emotional quality of life, diagnosis and treatment are often overlooked.
Commonly co-occurring urogenital functional diseases include overactive bladder syndrome, high-tone pelvic floor dysfunction, and vulvodynia. Other typical functional diseases are fibromyalgia, IBS, chronic fatigue syndrome, atopic allergy, and some rheumatic diseases. Thus, a multidisciplinary collaborative treatment approach among experts from multiple departments and sub-specialties is necessary.
A noteworthy point is that high-tone pelvic floor dysfunction is concomitantly present in 70%–94% of IC/BPS cases and can be identified by voiding dysfunction in UDS or pelvic pain recurring when the pelvic floor muscles are stimulated on a physical examination of the pelvic floor area.
The MAPP research network presents 2 patient groups with distinctive pain phenotypes in IC/BPS: (1) patients who complain only of pelvic pain and (2) patients who report pain in a broader area, as well as pelvic pain. Over 75% of patients are included in the second group, and this statistical distribution can furnish epidemiological evidence supporting the theory that complex pain originates from the central nervous system in the form of neuropathic pain with central sensitization mechanisms.
Hunner lesions, formerly called Hunner ulcers, are a pathognomonic finding of classical IC, which accounts for a minority of IC/BPS patients. It can be easily identified on cystoscopy, and several additional imaging modalities, such as filling the bladder beyond the functional bladder capacity or fluorescent endoscopy, have been used for easier identification. However, subsequent studies have suggested that BPS is a clinical syndrome in the form of a symptom complex and distinct from the minor specific patient groups with Hunner lesions. In addition, in IC/BPS patients who have been diagnosed with a clinical syndrome from a symptomatic perspective, no significant difference exists according to the presence or absence of Hunner lesions and manifestation of clinical symptoms. The progression of the disease involves repeated cycles of waxing and waning and remission and recurrence, and patients undergo a chronic prognostic process. In this process, many patients may experience abrupt and severe exacerbation of symptoms (or flare-ups) for several days. Common risk factors include certain foods, excessive physical activity, sexual contact, and exercise.
In some patients, the diagnostic definition of IC/BPS is sometimes consistent with chronic prostatitis/CPPS, which is NIH category III frequently occurring in men.
In the initial interview with patients, since the preemptive occurrence of prodromal symptoms associated with pelvic pain and irritative symptoms frequently occurs for several to 10 years due to the characteristics of the disease, clinicians should note the presence or absence of prodromal symptoms. The average age of onset of the disease is 40 years old, and onset of symptoms in pediatric patients is very rare. Regarding the epidemiological characteristics specified of family history, the incidence is about 17 times higher in parents, siblings, and children of affected adult women than in the general population.
Ketamine abuse is not a major medical issue in South Korea, but it is an issue associated with IC/BPS in some countries. Although ketamine abuse can occur in all age groups, clinicians should be particularly cautious in the diagnosis when younger patients show the symptomatic combination of IC/BPS regardless of bladder inflammation.
Currently, there is no histological definition of IC/BPS with diagnostic specificity. Although 24%–76% of patients show inflammatory histopathology, most appear to be chronic inflammatory changes without specificity. Hunner lesions can be specified as dense deposits of lymphoplasmacytes on the bladder epithelium throughout the specimen. The significance of a histological examination using cystoscopy is to exclude malignant diseases such as carcinoma in situ and other specific pathological diagnoses. Although recent studies have suggested various perspectives on the causes of IC/BPS symptoms, they can be summarized into 2 perspectives involving minor pathological processes and major pathological processes, respectively: (1) a local pathological process centered on local and target organs that considers neurogenic inflammation as a cause leading to activation of abnormal mast cells due to leakage of the GAG layer of the bladder epithelium in a small group of patients; and (2) a broad pathological process due to cross-sensitization of organs in the pelvis when the symptoms can be explained by comorbid widespread pain syndrome or complex regional pain syndrome, and immune-mediated pain in a larger group of patients. Pain, pressure sense, and discomfort occur according to the decrease in bladder capacity and bladder filling in IC patients, but bladder compliance is known to be normal on cystometry. Failure to diagnose or overlook causes of pelvic pain that can be overlooked regardless of IC/BPS, such as high-tone pelvic floor dysfunction and vulvodynia, which can be considered a gynecological disease, are the major causes of inadequate treatment of pelvic pain symptoms. Microscopic hematuria and pyuria are not significant predictive factors for Hunner lesions. Therefore, if a Hunner lesion is suspected or there is no response to primary empirical symptom treatment, clinicians should not hesitate to perform an endoscopic examination.
Although bladder hydrodistension is not included in the standard diagnostic process, recent studies have classified anesthetic low bladder capacity appearing in anesthetic bladder hydrodistension as a phenotype with a separate clinical progression. The studies also suggest that anesthetic low bladder capacity tends to appear in patients with more severe symptoms, a low frequency of comorbid diseases such as depression, and IBS beyond the urogenital area, and symptoms that mainly target the bladder. Moreover, since glomerulation (punctiform subepithelial or submucosal hemorrhages) appearing in bladder hydrodistension has low sensitivity and specificity, it is not necessary for the diagnosis.
Treatment for early-stage disease is usually conservative. Prior to treatment, patients should be educated on the disease and specificity of the treatment process. After that, treatment involves dietary control by understanding and sequentially avoiding certain foods and beverages that have negative effects on symptom control, use of versatile nonprescription analgesics, and relaxation training of pelvic floor muscles through physiotherapy. Conservative drug treatment complies with a method of sequentially controlling intensity according to symptom control. At the discretion of medical staff, intensive treatment can alternatively be considered when a patient shows severe symptoms in the initial stage. The symptoms may be further aggravated by increased sensitivity to pain due to patients’ irrational and catastrophic thinking and obsession with or fear of miserable results. This can be controlled sufficiently with cognitive behavioral therapy. Currently, the U.S. Food and Drug Administration (FDA) has approved only 2 medications to treat IC/BPS symptoms: PPS as an oral drug and dimethyl sulfoxide as an intravesical injection drug. Amitriptyline, a tricyclic antidepressant, is not approved by the FDA, but is a major oral drug due to its antihistamine properties, ability to promote sleep, and its effect on relieving central and peripheral neuropathic pain. Although intravesical injection drugs are positively helpful for symptom control, they are limited for pain control according to the increased sensitivity in the nervous system due to the nature of local therapy on the target organs. In addition, no studies have confirmed the effects of the frequency of drug injection, drug maintenance time, and maintenance therapy. Regarding botulinum toxin A, ischuria is not common after this fourthline treatment. However, treatment should be chosen with caution in patients with obstructive voiding dysfunction. Major reconstructive surgery is rare but can be attempted when all other treatments have failed. Attempts have even been made to perform urinary diversion and cystectomy simultaneously for patients with obvious pathological findings such as Hunner lesions, severely low bladder compliance, and decreased bladder capacity. However, clinicians and patients considering this option should keep in mind that major reconstructive surgery does not guarantee the disappearance of pain. Furthermore, since continent urinary diversion surgery such as substitution cystoplasty, ileal conduit urinary diversion, Indiana pouch reservoir, and neobladder-to-urethra diversion uses a part of the small intestine, clinicians and patients should be aware when considering surgery that pain originating from the intestine and caused by the contraction of the substituted intestine cannot be excluded.
FINAL OPINIONS
We have read the article by Hanno about the definition of terms and new suggestions. At that time, we thought that the definition of terms and opinions were ambiguous for specifying or defining actual clinical situations. Now, more than ever, we believe that these implications and ambiguities should be viewed as characteristics of a condition that is defined as a combination of distinctive symptoms. Moreover, in the absence of reliable, high-quality clinical studies due to the clinical heterogeneity of IC/BPS, a reasonable animal model that represents the complexity of the disease should be established in basic research. In that case, advances should be made in standardized research, which is essential as part of clinical research that can characterize the phenotypes of various clinical situations and in reproducible and valid laboratory-based translational research. The most important development covered in this review is the epidemiological, pathological, and (more notably) distinct identifiability of the prognostic value of various treatments for Hunner lesions.
In all clinical fields, diagnoses and treatments specific to each symptom are performed, and this approach is known to be reasonable. However, patients and clinicians should be aware that this approach is very time-consuming and it is an arduous process to listen to the patients’ expressions of symptoms, to educate patients on the perception of the disease, and to form a relationship with patients for treatment. Only through these simple but laborious humane efforts can medical staff improve patients’ quality of life with the abovementioned treatments or in more effective ways. We hope that medical staff have confidence and welcome patients.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTION STATEMENT
Conceptualization: KHK
Data curation: SJK
Formal analysis: SJK
Writing - original draft: KHK
Writing - review & editing: SJK, KHK