 |
 |


- Search
| Int Neurourol J > Volume 26(4); 2022 > Article |
|
ABSTRACT
Purpose
Substantive evidence supports a role of chronic stress in the development, maintenance, and even enhancement of functional bladder disorders such as interstitial cystitis/bladder pain syndrome (IC/BPS). Increased urinary frequency and bladder hyperalgesia have been reported in rodents exposed to a chronic stress paradigm. Here, we utilized a water avoidance stress (WAS) model in rodents to investigate the effect of chronic stress on vascular perfusion and angiogenesis.
Methods
Female Wistar-Kyoto rats were exposed to WAS for 10 consecutive days. Bladder neck tissues were analyzed by western immunoblot for vascular endothelial growth factor (VEGF) and nerve growth factor precursor (proNGF). Vascular perfusion was assessed by fluorescent microangiography followed by Hypoxyprobe testing to identify regions of tissue hypoxia.
Results
The expression of VEGF and proNGF in the bladder neck mucosa was significantly higher in the WAS rats than in the controls. There was a trend toward increased vascular perfusion, but without a statistically significant difference from the control group. The WAS rats displayed a 1.6-fold increase in perfusion. Additionally, a greater abundance of vessels was observed in the WAS rats, most notably in the microvasculature.
Conclusions
These findings show that chronic psychological stress induces factors that can lead to increased microvasculature formation, especially around the bladder neck, the region that contains most nociceptive bladder afferents. These findings may indicate a link between angiogenesis and other inflammatory factors that contribute to structural changes and pain in IC/BPS.
The lower urinary tract has 2 main functions—namely, storing urine until the capacity of the bladder is reached and micturition once an appropriate time and place are found to empty the bladder. The proper execution of these functions is dependent on a delicate interplay between local and central processes, which convey sensory information to our awareness and allow us to voluntarily initiate micturition. Lower urinary tract symptoms (LUTS) comprise bothersome problems experienced during the storage and/or voiding of urine. LUTS are highly prevalent in the general population and have a major impact on patients’ quality of life [1]. Clinical studies and population-based surveys have indicated that a strong relationship exists between LUTS and symptoms related to anxiety and depression, which often manifest as comorbid conditions [2,3]. This relationship appears to be of a bidirectional nature and has been established for a variety of LUTS and syndromes including urinary frequency, overactive bladder, and interstitial cystitis/bladder pain syndrome (IC/BPS) [4,5]. This paper aimed to further elucidate the relationship between chronic psychological stress and IC/BPS.
IC/BPS is characterized as a chronic condition presenting with chronic pelvic pain that is perceived to be localized to the bladder and is accompanied by storage dysfunction. The mechanisms underlying this condition are still largely unknown. However, chronic psychological stress and anxiety may initiate maladaptive changes in nociceptive pathways, ultimately resulting in stress-induced hyperalgesia and tactile allodynia [6]. Studies have indicated that increased stress is highly prevalent in IC/BPS patients and is associated with the exacerbation of symptoms [7-11]. Therapeutic interventions aimed at improving psychological well-being have also been reported to reduce IC/BPS symptoms [12,13]. It is likely that the stress-related exacerbation of IC/BPS is associated with maladaptive changes in nociceptive pathways.
Experimental models in which anxiety-prone rodents are exposed to a chronic stress paradigm induce symptoms associated with IC/BPS, such as severe anxiety-related behavior, tactile allodynia in the suprapubic region, and a significant increase in voiding frequency [14-19]. A frequently used model to induce chronic psychological stress in studies investigating the relationship with lower urinary tract function is the water avoidance stress (WAS) paradigm. Previous studies utilizing WAS in rats to investigate IC/BPS-associated changes in lower urinary tract function have indicated that exposure to WAS leads to visceral hypersensitivity during bladder filling and alterations in the central nervous system response [18].
IC/BPS is associated with increased expression of vascular endothelial growth factor (VEGF), an essential regulator of angiogenesis and vascular permeability crucial for the maintenance of healthy vascular function [20]. The high expression of VEGF that can be observed in IC/BPS is associated with immature angiogenesis, leading to fragile and in some cases hemorrhage-prone vessels, and VEGF levels show a positive relationship with pain severity in patients suffering from IC/BPS [21, 22]. VEGF is recognized as a survival factor for endothelial cells and other cell types [23], such as renal tubular epithelial cells [24]. It has been suggested that the increase in VEGF initially acts a survival mechanism in response to IC/BPS, but can lead to detrimental effects, such as edema and inflammation [25,26].
In addition to its role in the maintenance of vascular function, research has suggested that VEGF plays an important role in neuroprotective processes, and reduced levels of VEGF may promote neuronal degeneration [27]. It has been proposed that VEGF exerts neuroplastic effects by playing a modulating role in nerve growth factor (NGF) signaling pathways, and VEGF inhibition has been shown to induce a decrease in the expression of proNGF, the precursor of NGF [28]. Urinary NGF levels have been reported to show a direct association with IC/BPS symptom severity and treatment efficacy [29,30]. In addition to its neuroplastic and neuroprotective effects, proNGF plays a mediating role in the induction of angiogenic processes [31].
In the current study, we utilized WAS-induced chronic stress in rats as a model for IC/BPS and investigated levels of VEGF and proNGF along with the assessment of vascular perfusion and angiogenesis in the region of the bladder neck. We hypothesized that exposing rats to a WAS paradigm would induce an elevation of VEGF and proNGF levels in the bladder neck and would have an impact on vascular perfusion and angiogenesis.
This study utilized young adult female Wistar-Kyoto (WKY) rats (180–200 g) purchased from a commercial vendor (Charles River Laboratories International, Inc. Wilmington, MA, USA). This particular strain is genetically predisposed to elevated levels of anxiety [32] and has successfully been used to study stress-induced visceral hyperalgesia in earlier studies [17]. Rats were assigned to either WAS or control conditions at random. Animals were kept in standard housing with 12-hour light/dark cycles and ad libitum access to standard chow and water. The Institutional Animal Care and Use Committee approved all procedures, which conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Rats were divided into WAS and control groups at random. The WAS paradigm utilized in this study was established [17] and hence used by the Multidisciplinary Approach to the Study Chronic Pelvic Pain (MAPP) research network [18]. In short, rats were placed on a pedestal (8 cm×8 cm×11.5 cm) in a water-filled container (1 hr/day for 10 consecutive days) and sacrificed on day 11. Controls were handled in the same way as the WAS rats but placed in a clean cage (1 hr/day for 10 consecutive days). All procedures for both groups were conducted in the morning to control for circadian effects. The animals were sacrificed by exsanguination during isoflurane anesthesia.
Following removal, bladder tissue was placed in a Sylgard-lined dissecting dish and cut open longitudinally to isolate the region around the bladder neck for further processing. After surgically separating the urinary mucosa (consisting of the urothelium and lamina propria) from the smooth muscle, tissue was homogenized using Lysing Matrix D in a FastPrep 24 instrument (MP Biomedicals, Solon, OH, USA) in HBSS (5mM KCl, 0.3mM KH2PO4, 138mM NaCl, 4mM NaHCO3, 0.3mM Na2HCO3, 0.3mM Na2HPO4, 5.6mM glucose, 10mM HEPES, 1mM DL-dithiothreitol, and 1mM ethylenediaminetetraacetic acid; pH, 7.4) containing a complete protease inhibitor cocktail (1 tablet/10 mL; Roche, Indianapolis, IN, USA) and phosphatase inhibitor cocktail (1:100; Sigma-Aldrich, St. Louis, MO, USA). After centrifugation (16,200×g; 15 min at 4°C), we suspended the membrane pellets in lysis buffer containing 0.3M NaCl, 50mM Tris-HCl (pH, 7.6), 0.5% Triton X-100, and the same concentration of protease inhibitors as above to prepare the membrane protein fraction. We then incubated the suspensions on ice followed by centrifugation (16,200 ×g; 15 minutes at 4°C). The supernatants were combined, and total protein concentrations were assessed using the Pierce BCA protein assay (Thermo Scientific, Rockford, IL, USA). After denaturation (100°C for 5 min) in the presence of Laemmli sample buffer, lysate from each sample was separated on an Any kD TGX Stain-Free SDS-PAGE gel (Bio-Rad Laboratories, Hercules, CA, USA). As a loading control, the total protein per sample was determined using Bio-Rad Stain-Free SDS-PAGE gel technology. Ultraviolet-activated protein fluorescence was imaged on a ChemiDoc MP (Bio-Rad Laboratories). After proteins were transferred to polyvinylidene fluoride membranes, the membranes were incubated in 5% (w/v) dried milk dissolved in tris buffered saline with tween (TBS-T) (20mM Trizma, 137mM NaCl, 0.1% Tween-20; pH, 7.6), rinsed with TBS-T, and incubated overnight at 4°C with primary antibodies for VEGFA (AB46154, 1:1,000 in 5% milk; Abcam, Cambridge, MA, USA [WAS, N =10; control, N =11]) and proNGF (OSN00007G, 1:1,000 in 5% Milk; Life Technologies, Carlsbad, CA, USA [WAS, N =6; control, N =6]). After washing in TBS-T, the membranes were incubated with a secondary antibody (donkey anti-rabbit HRP, GE Healthcare, Marlborough, MA, USA) or goat anti-rabbit IgG HRP (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) for 1 hour in 5% (w/v) milk TBS-T, washed, incubated in WesternBright Quantum (Advansta, Menlo Park, CA, USA) and then imaged on a ChemiDoc MP (Bio-Rad Laboratories). A single immunoreactive band was observed for both VEGF (45 kDa) and proNGF (54 kDa). The optical density of each protein species was determined and normalized to the total protein using Image Lab software (Bio-Rad Laboratories).
The control and WAS rats were subjected to fluorescent microangiography (N=3 per group) as previously described [33,34]. In brief, deeply anesthetized rats received an intracardiac injection of low-temperature wax (1% low melting agarose gel; Sigma-Aldrich) containing fluorescent beads (5 mL of 1% FluoSpheres Carboxylate-Modified Microspheres 0.02 μm; Life Technologies, Carlsbad, CA, USA). Following the infusion with low melting temperature wax and beads, the bladders were excised and placed on ice for 10 minutes before fixation with 4% paraformaldehyde.
Two hours before tissue harvest, injection of the gold-standard immunohistochemical hypoxia marker Hypoxyprobe (Pimonidazole HCl; Hypoxyprobe, Burlington, MA, USA) was done to visualize areas of tissue hypoxia. The bladders were then processed into frozen blocks, exhaustively sectioned, and then imaged along with the fluorescent microbeads. These images were then quantitated using MATLAB as previously described [33,34], and a high-throughput analysis was performed to assess regional changes in blood flow and vascular networks (e.g., microvessel abundance and area).
The results are presented as mean±standard error of the mean. The values for the control versus WAS groups were compared using the 2-tailed unpaired Student t-test for parametrically distributed data or the Mann-Whitney test for nonparametric data sets, using GraphPad Prism 7 (GraphPad Software, La Jolla, CA, USA). P-values<0.05 were considered statistically significant.
We first assessed whether VEGF protein expression was higher in bladder neck mucosa obtained from rats exposed to WAS compared to the control group using western immunoblotting. VEGF expression is known to be elevated in IC/BPS, and VEGF is involved in angiogenic processes. Bladder neck mucosa VEGF protein expression was significantly higher by 1.8-fold in the WAS rats than in the control rats (80.81±10.6, N=11 vs. 44.14±7.96, N=10, P=0.0156) (Fig. 1A).
Elevated levels of VEGF are associated with increased expression of proNGF, which has been reported to be involved in neuroplastic changes in cystitis and mediates proangiogenic processes. Western blot analysis indicated that levels of proNGF were significantly higher, by 2.6-fold, in bladder neck mucosa from WAS animals than in controls (3.38 ±0.47, N =6 vs. 1.28±0.16, N=6, P=0.0017) (Fig. 1B).
We then perfused the urinary bladder of the control and WAS rats with low-melting-temperature agar containing fluorescent beads. There was a trend toward increased vasculature perfusion in WAS rat bladders (Fig. 2A, green depicts regions of perfusion throughout the bladder neck mucosa/submucosa, N=3 per group). Perfusion efficiency was calculated by measuring the total blood vessel area, defined as the area containing fluorescent beads. This region was then expressed as a percentage of the total tissue area. The WAS rats showed a 1.6-fold increase in vascular perfusion. Representative micrographs from the control and WAS bladders are shown in Fig. 2A. The quantitative analysis showed a more variable distribution in the bladders from WAS rats versus a more narrow distribution in the control bladders. However, the calculated perfusion ratio in bladders from the WAS rats (4.42±1.12, N=3) and control rats (2.78±0.16, N=3) did not significantly differ (P=0.22) (Fig. 2B). Although genes implicated in the regulation of angiogenesis, such as VEGF, are known to be regulated by hypoxia, we found no obvious signs of tissue hypoxia in either the control or WAS bladders, as the intensity of staining of pimonidazole adducts was similar in both WAS and control bladder tissue. We also observed a greater abundance of vessels in the WAS rats (especially the microvasculature).
In the present study, we examined the effects of a chronic psychological stress paradigm on inducing symptoms related to IC/BPS in the bladder neck mucosa. We provide support to the proposal that exposure to chronic psychological stress can alter bladder health at a structural level. Markers related to angiogenesis, inflammatory, and neuroprotective processes were significantly increased in WAS rats. The exacerbated expression of these factors, over time, could lead to dysfunctional alterations in nociceptive pathways and contribute to the clinical phenotype of IC/BPS.
The mucosal samples obtained from the bladder neck region of rats exposed to a WAS paradigm showed a significantly higher expression of VEGF compared to the control group. The overexpression of VEGF in WAS rats indicates the initiation of angiogenic processes in the bladder neck. This culminates in immature blood vessels, which are vulnerable to become leaky and potential regions of ischemia, and is an important factor involved in the development of edema and inflammation regularly seen in IC/BPS [25,35]. VEGF has been shown to be involved in bladder wall permeability and deterioration of the barrier function of the urothelium [36,37]. Deterioration of the barrier function of the urothelium allows urea and toxins present in the harsh chemical composition of urine to pass into the underlying layers of the bladder and has been linked to sensations of urgency and pain during the filling phase, as well as urinary frequency [38]. VEGF is involved in neuroplastic changes in IC/BPS related to hypersensitivity in response to noxious stimuli and, interestingly, evidence indicates that cystitis-induced neuroplasticity in the bladder can be prevented by the administration of VEGF-neutralizing antibodies [39], showing a direct modulatory effect of VEGF on the peripheral nervous system. Targeting VEGF expression by administering anti-VEGF-neutralizing antibodies has also been shown to significantly reduce the nociceptive response to a model of acute cystitis [40]. Because of its established expression in IC/BPS and correlation with symptom severity [21], the MAPP research network has suggested that VEGF might serve as a useful biomarker to diagnose the presence and potentially the severity of IC/BPS in bladder biopsies [22].
An important role for neurotrophins, such as NGF, as versatile signaling molecules in allostatic adaptations to exposure to stressors has been established [41]. In the central nervous system, the expression of neurotrophins is significantly altered after exposure to stressors. Altered neurotrophin expression initiates neurobiological changes that are associated with the pathophysiology of affective disorders. In particular, in the hypothalamic-pituitary-adrenal axis, maladaptive changes can be observed after exposure to stress-inducing stimuli. Furthermore, NGF has been indicated to play an essential role in nociceptive processing [42]. The bladder neck mucosa in WAS rats showed a significant increase in the expression of proNGF, as measured by western blot analysis. These results are in agreement with previously reported elevations in NGF (the mature form of proNGF) in patients with IC/BPS [30,43]. The promotion of neuronal survival and differentiation by NGF is carried out by binding to tropomyosin receptor kinase A (TrkA) and p75 neurotrophin receptor (p75ntr). A delicate balance of NGF binding to both TrkA and p75ntr receptors is required for neuroprotective and differentiation-related activity. Binding to p75ntr receptors in the absence of binding to TrkA induces apoptosis and axonal pruning [44]. ProNGF can, by selective binding to p75ntr but not TrkA, evoke apoptotic processes [45,46]. Given the deterioration of urothelial health in IC/BPS [47], the accumulation of proNGF and the associated onset of apoptotic processes are likely to negatively impact the restorative capacity of the urothelium, resulting in a further decline of lower urinary tract health and potentially an exacerbation of IC/BPS-related symptoms.
NGF is a notable mast cell activator, and the accumulation of NGF could play a key role in mast cell activation in IC/BPS [48]. IC/BPS is known as a chronic inflammatory disease, and increased mast cell activation has been postulated to play a role in its onset and progression [49,50]. Mediators secreted by mast cells may explain the symptoms of IC/BPS related to inflammatory processes, angiogenesis, and neuronal hyperexcitability in the bladder wall. Mast cell activation in the bladder has been reported in rodents exposed to a 10-day WAS protocol [17], and the increased release of VEGF in an acute stress paradigm has, interestingly, been indicated to be regulated through a mast cell-dependent process [37].
The developmental processes of blood vessels and nerves share many common signaling pathways [51] and studies on retinal tissue have suggested that proNGF is likely to be involved in angiogenic and inflammatory processes [31,52]. Studies have indicated that proNGF and NGF initiate angiogenic processes via TrkA binding and downstream signaling pathways such as PI3K and ERK [53]. Inflammatory processes in IC/BPS may culminate in increased vascular perfusion and permeability, which are associated with peripheral edema [54].
Although not statistically significant, we report a trend towards an increase in vascular perfusion in WAS rats, and the microvasculature also appeared to be increased in WAS rats. These changes are likely due to the direct and indirect effects of the increased expression of VEGF and proNGF on angiogenic processes and vascular permeability. Quantification of vascular perfusion in the bladder neck is methodologically challenging, and larger N-numbers might be required to accurately reflect potential changes in perfusion related to IC/BPS in this animal model. The observed vascular increases, in particular in the microvasculature, could indicate the presence of immature blood vessels. Immature angiogenesis, leading to fragile vessels and potential areas of ischemia, has previously been reported in IC/BPS and is linked to an increase in VEGF expression [21,39]. Previous research has suggested that increased VEGF expression in IC/BPS might be caused by hypoxia in the bladder [55,56], but our data did not show evidence of hypoxia in WAS rats. This might indicate that NGF-induced mast cell activation plays a more prominent role in the increased release of VEGF [37].
To conclude, in the present work we showed that exposing rats to a 10-day WAS protocol led to a significant increase in the expression of VEGF and proNGF in the bladder neck mucosa, induced a trend towards an increase in vascular perfusion, and promoted a greater abundance of vessels (particularly in the microvasculature). We propose that these detrimental structural changes in the lower urinary tract negatively impact overall lower urinary tract health, as well as urothelial signaling and barrier functions, and play an important role in the clinical phenotype seen in IC/BPS. The current study solely focused on changes in the bladder neck associated with IC/BPS. More work is needed to determine whether the observed changes occur in the complete bladder or are isolated to the bladder neck. Future research should aim to assess the effects of duration of WAS exposure on these outcome measures, integrate the assessment of tissue markers associated with psychological stress, and further investigate the relationships between VEGF and proNGF expression and processes related to angiogenesis, neuroplasticity, and apoptosis with the potential of identifying and improving biomarkers of IC/BPS and further uncovering mechanisms that can be utilized to improve therapeutic options for IC/BPS patients.
NOTES
Research Ethics
The Institutional Animal Care and Use Committee approved all procedures, which conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
AUTHOR CONTRIBUTION STATEMENT
·Conceptualization: LVR, LAB
·Data curation: AWJ, AFK, KM, SSL, LVR
·Formal analysis: AWJ, AFK, KM, SSL, LVR, LAB
·Funding acquisition: LAB, LVR
·Methodology: AWJ, AFK, KM, SSL, LVR
·Project administration: LAB
·Visualization: MDR, KM, LAB
·Writing - original draft: MDR, LAB
·Writing - review & editing: MDR, AWJ, SSL, GAVK, LVR, LAB
REFERENCES
1. Coyne KS, Wein AJ, Tubaro A, Sexton CC, Thompson CL, Kopp ZS, et al. The burden of lower urinary tract symptoms: evaluating the effect of LUTS on health-related quality of life, anxiety and depression: EpiLUTS. BJU Int 2009;103 Suppl 3:4-11. PMID: 19302497


2. Vrijens D, Berghmans B, Nieman F, van Os J, van Koeveringe G, Leue C. Prevalence of anxiety and depressive symptoms and their association with pelvic floor dysfunctions-A cross sectional cohort study at a Pelvic Care Centre. Neurourol Urodyn 2017;36:1816-23. PMID: 28220586



3. Clemens JQ, Mullins C, Kusek JW, Kirkali Z, Mayer EA, Rodríguez LV, et al. The MAPP research network: a novel study of urologic chronic pelvic pain syndromes. BMC Urol 2014;14:57. PMID: 25085007




4. Vrijens D, Drossaerts J, van Koeveringe G, Van Kerrebroeck P, van Os J, Leue C. Affective symptoms and the overactive bladder-a systematic review. J Psychosom Res 2015;78:95-108. PMID: 25499886


5. Perry S, McGrother CW, Turnerr K; Leicestershire MRC Incontinence Study Group. An investigation of the relationship between anxiety and depression and urge incontinence in women: development of a psychological model. Br J Health Psychol 2006;11(Pt 3):463-82. PMID: 16870056


6. Jennings EM, Okine BN, Roche M, Finn DP. Stress-induced hyperalgesia. Prog Neurobiol 2014;121:1-18. PMID: 25010858


7. Rothrock NE, Lutgendorf SK, Kreder KJ, Ratliff T, Zimmerman B. Stress and symptoms in patients with interstitial cystitis: a life stress model. Urology 2001;57:422-7. PMID: 11248609


8. Nickel JC, Tripp DA, Pontari M, Moldwin R, Mayer R, Carr LK, et al. Psychosocial phenotyping in women with interstitial cystitis/ painful bladder syndrome: a case control study. J Urol 2010;183:167-72. PMID: 19913812


9. Rothrock NE, Lutgendorf SK, Hoffman A, Kreder KJ. Depressive symptoms and quality of life in patients with interstitial cystitis. J Urol 2002;167:1763-7. PMID: 11912405


10. Clemens JQ, Brown SO, Calhoun EA. Mental health diagnoses in patients with interstitial cystitis/painful bladder syndrome and chronic prostatitis/chronic pelvic pain syndrome: a case/control study. J Urol 2008;180:1378-82. PMID: 18707716



11. Naliboff BD, Stephens AJ, Lai HH, Griffith JW, Clemens JQ, Lutgendorf S, et al. Clinical and psychosocial predictors of urological chronic pelvic pain symptom change in 1 year: a prospective study from the MAPP Research Network. J Urol 2017;198:848-57. PMID: 28528930



12. O’Hare PG 3rd, Hoffmann AR, Allen P, Gordon B, Salin L, Whitmore K. Interstitial cystitis patients’ use and rating of complementary and alternative medicine therapies. Int Urogynecol J 2013;24:977-82. PMID: 23149598



13. Kanter G, Komesu YM, Qaedan F, Jeppson PC, Dunivan GC, Cichowski SB, et al. Mindfulness-based stress reduction as a novel treatment for interstitial cystitis/bladder pain syndrome: a randomized controlled trial. Int Urogynecol J 2016;27:1705-11. PMID: 27116196




14. Lee UJ, Ackerman AL, Wu A, Zhang R, Leung J, Bradesi S, et al. Chronic psychological stress in high-anxiety rats induces sustained bladder hyperalgesia. Physiol Behav 2015;139:541-8. PMID: 25449389


15. Robbins M, DeBerry J, Ness T. Chronic psychological stress enhances nociceptive processing in the urinary bladder in high-anxiety rats. Physiol Behav 2007;91:544-50. PMID: 17521683



16. Ness TJ, Randich A, Nelson DE, Su X. Screening and optimization of nerve targets and parameters reveals inhibitory effect of pudendal stimulation on rat bladder hypersensitivity. Reg Anesth Pain Med 2016;41:737-43. PMID: 27685349


17. Smith AL, Leung J, Kun S, Zhang R, Karagiannides I, Raz S, et al. The effects of acute and chronic psychological stress on bladder function in a rodent model. Urology 2011;78:967.e1-7. PMID: 21868072



18. Wang Z, Chang HH, Gao Y, Zhang R, Guo Y, Holschneider DP, et al. Effects of water avoidance stress on peripheral and central responses during bladder filling in the rat: a multidisciplinary approach to the study of urologic chronic pelvic pain syndrome (MAPP) research network study. PLoS One 2017;12:e0182976. PMID: 28886046



19. Bradesi S, Schwetz I, Ennes HS, Lamy CM, Ohning G, Fanselow M, et al. Repeated exposure to water avoidance stress in rats: a new model for sustained visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol 2005;289:G42-53. PMID: 15746211


20. Tamaki M, Saito R, Ogawa O, Yoshimura N, Ueda T. Possible mechanisms inducing glomerulations in interstitial cystitis: relationship between endoscopic findings and expression of angiogenic growth factors. J Urol 2004;172:945-8. PMID: 15311005


21. Kiuchi H, Tsujimura A, Takao T, Yamamoto K, Nakayama J, Miyagawa Y, et al. Increased vascular endothelial growth factor expression in patients with bladder pain syndrome/interstitial cystitis: its association with pain severity and glomerulations. BJU Int 2009;104:826-31 discussion 831. PMID: 19298410


22. Dagher A, Curatolo A, Sachdev M, Stephens AJ, Mullins C, Landis JR, et al. Identification of novel non-invasive biomarkers of urinary chronic pelvic pain syndrome: findings from the Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network. BJU Int 2017;120:130-42. PMID: 28263447




23. Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, Rangell L, et al. VEGF is required for growth and survival in neonatal mice. Development 1999;126:1149-59. PMID: 10021335



24. Kanellis J, Fraser S, Katerelos M, Power DA. Vascular endothelial growth factor is a survival factor for renal tubular epithelial cells. Am J Physiol Renal Physiol 2000;278:F905-15. PMID: 10836978


25. Saban R. Angiogenic factors, bladder neuroplasticity and interstitial cystitis-new pathobiological insights. Transl Androl Urol 2015;4:555-62. PMID: 26816854


26. Clemens JQ, Mullins C, Ackerman AL, Bavendam T, van Bokhoven A, Ellingson BM, et al. Urologic chronic pelvic pain syndrome: insights from the MAPP Research Network. Nat Rev Urol 2019;16:187-200. PMID: 30560936




27. Storkebaum E, Lambrechts D, Carmeliet P. VEGF: once regarded as a specific angiogenic factor, now implicated in neuroprotection. Bioessays 2004;26:943-54. PMID: 15351965


28. Segatto M, Fico E, Gharbiya M, Rosso P, Carito V, Tirassa P, et al. VEGF inhibition alters neurotrophin signalling pathways and induces caspase-3 activation and autophagy in rabbit retina. J Cell Physiol 2019;234:18297-307. PMID: 30891770



29. Tonyali S, Ates D, Akbiyik F, Kankaya D, Baydar D, Ergen A. Urine nerve growth factor (NGF) level, bladder nerve staining and symptom/problem scores in patients with interstitial cystitis. Adv Clin Exp Med 2018;27:159-63. PMID: 29521057


30. Liu HT, Tyagi P, Chancellor MB, Kuo HC. Urinary nerve growth factor level is increased in patients with interstitial cystitis/bladder pain syndrome and decreased in responders to treatment. BJU Int 2009;104:1476-81. PMID: 19522864


31. Elshaer SL, Abdelsaid MA, Al-Azayzih A, Kumar P, Matragoon S, Nussbaum JJ, et al. Pronerve growth factor induces angiogenesis via activation of TrkA: possible role in proliferative diabetic retinopathy. J Diabetes Res 2013;2013:432659. PMID: 23998130




32. Paré WP. The performance of WKY rats on three tests of emotional behavior. Physiol Behav 1992;51:1051-6. PMID: 1615043


33. Mukherjee E, Maringer K, Papke E, Bushnell D, Schaefer C, Kramann R, et al. Endothelial marker-expressing stromal cells are critical for kidney formation. Am J Physiol Renal Physiol 2017;313:F611-20. PMID: 28539333



34. Kramann R, Tanaka M, Humphreys BD. Fluorescence microangiography for quantitative assessment of peritubular capillary changes after AKI in mice. J Am Soc Nephrol 2014;25:1924-31. PMID: 24652794



35. Erickson DR, Belchis DA, Dabbs DJ. Inflammatory cell types and clinical features of interstitial cystitis. J Urol 1997;158(3 Pt 1):790-3. PMID: 9258082


36. Saban R, Saban MR, Maier J, Fowler B, Tengowski M, Davis CA, et al. Urothelial expression of neuropilins and VEGF receptors in control and interstitial cystitis patients. Am J Physiol Renal Physiol 2008;295:F1613-23. PMID: 18815217



37. Cao J, Boucher W, Kempuraj D, Donelan JM, Theoharides TC. Acute stress and intravesical corticotropin-releasing hormone induces mast cell dependent vascular endothelial growth factor release from mouse bladder explants. J Urol 2006;176:1208-13. PMID: 16890727


39. Saban MR, Davis CA, Avelino A, Cruz F, Maier J, Bjorling DE, et al. VEGF signaling mediates bladder neuroplasticity and inflammation in response to BCG. BMC Physiol 2011;11:16. PMID: 22059553



40. Lai HH, Shen B, Vijairania P, Zhang X, Vogt SK, Gereau RW 4th. Anti-vascular endothelial growth factor treatment decreases bladder pain in cyclophosphamide cystitis: a Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network animal model study. BJU Int 2017;120:576-83. PMID: 28581681




41. Cirulli F, Alleva E. The NGF saga: from animal models of psychosocial stress to stress-related psychopathology. Front Neuroendocrinol 2009;30:379-95. PMID: 19442684


42. Barker PA, Mantyh P, Arendt-Nielsen L, Viktrup L, Tive L. Nerve growth factor signaling and its contribution to pain. J Pain Res 2020;13:1223-41. PMID: 32547184


43. Lowe EM, Anand P, Terenghi G, Williams-Chestnut RE, Sinicropi DV, Osborne JL. Increased nerve growth factor levels in the urinary bladder of women with idiopathic sensory urgency and interstitial cystitis. Br J Urol 1997;79:572-7. PMID: 9126085


44. Teng KK, Felice S, Kim T, Hempstead BL. Understanding proneurotrophin actions: recent advances and challenges. Dev Neurobiol 2010;70:350-9. PMID: 20186707



45. Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science 2001;294:1945-8. PMID: 11729324


46. Teng HK, Teng KK, Lee R, Wright S, Tevar S, Almeida RD, et al. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J Neurosci 2005;25:5455-63. PMID: 15930396



47. Jhang JF, Kuo HC. Pathomechanism of interstitial cystitis/bladder pain syndrome and mapping the heterogeneity of disease. Int Neurourol J 2016;20(Suppl 2):S95-104. PMID: 27915472




48. Sant GR, Kempuraj D, Marchand JE, Theoharides TC. The mast cell in interstitial cystitis: role in pathophysiology and pathogenesis. Urology 2007;69(4 Suppl):34-40. PMID: 17462477


49. Aich A, Afrin LB, Gupta K. Mast cell-mediated mechanisms of nociception. Int J Mol Sci 2015;16:29069-92. PMID: 26690128



50. Theoharides TC, Sant GR, el-Mansoury M, Letourneau R, Ucci AA Jr, Meares EM Jr. Activation of bladder mast cells in interstitial cystitis: a light and electron microscopic study. J Urol 1995;153(3 Pt 1):629-36. PMID: 7861501


51. Carmeliet P. Blood vessels and nerves: common signals, pathways and diseases. Nat Rev Genet 2003;4:710-20. PMID: 12951572



52. Mysona BA, Abdelsaid MA, Matragoon S, Pillai B, El-Remessy A. Inflammatory role of ProNGF/p75NTR in Müller cells of the diabetic retina. Investig Ophthalmol Vis Sci 2012;53:2003.
53. Romon R, Adriaenssens E, Lagadec C, Germain E, Hondermarck H, Le Bourhis X. Nerve growth factor promotes breast cancer angiogenesis by activating multiple pathways. Mol Cancer 2010;9:157. PMID: 20569463




54. Kim HJ. Update on the pathology and diagnosis of interstitial cystitis/bladder pain syndrome: a review. Int Neurourol J 2016;20:13-7. PMID: 27032552




55. Jiang YH, Jhang JF, Lee YK, Kuo HC. Low-energy shock wave plus intravesical instillation of botulinum toxin A for interstitial cystitis/ bladder pain syndrome: pathophysiology and preliminary result of a novel minimally invasive treatment. Biomedicines 2022;10:396. PMID: 35203604



56. Lee JD, Lee MH. Increased expression of hypoxia-inducible factor- 1α and vascular endothelial growth factor associated with glomerulation formation in patients with interstitial cystitis. Urology 2011;78:971.e11-8. PMID: 21813166


Fig. 1.
Bar graphs depicting water avoidance stress (WAS)-associated changes in levels of VEGF (WAS: 80.81±10.6, N=11, control: 44.14±7.96, N=10; P=0.016) (A) and proNGF (WAS: 3.38±0.47, N=6, control: 1.28±0.16, N=6; P=0.002) (B). Western blot expression bands and representative total protein images are included. VEGF, vascular endothelial growth factor; proNGF, nerve growth factor precursor. *P≤0.05. **P≤0.01.
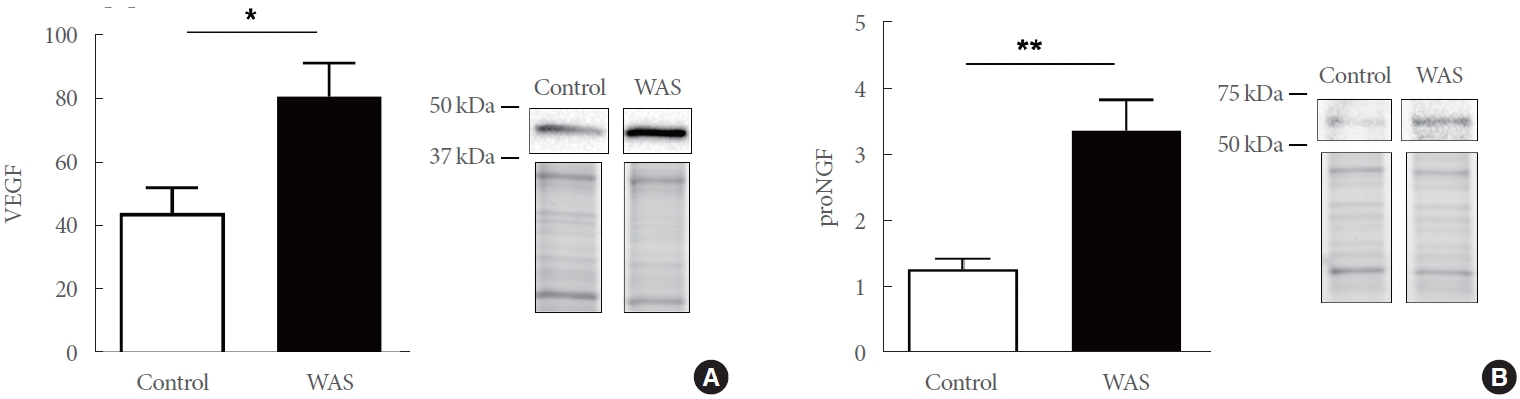
Fig. 2.
(A) Representative micrographs from control and water avoidance stress (WAS) bladder regarding tissue perfusion and hypoxia are shown. Green depicts regions of perfusion throughout the bladder neck mucosa/submucosa, N=3 per group. The WAS rats displayed an increase in perfusion. No obvious signs of tissue hypoxia in either control or WAS bladders can be observed, as the intensity of staining of pimonidazole adducts was similar in both WAS and control bladder tissue. (B) WAS rats showed a 1.6-fold increase in vascular perfusion. The calculated perfusion ratio in bladders from WAS (4.42±1.12, N=3) and control (2.78±0.16, N=3) animals did not significantly differ (P=0.22).






