Updates of Overactive Bladder in Pediatrics
Article information
Abstract
Overactive bladder (OAB) is clinically defined as urinary urgency with or without urinary incontinence. It is associated with daytime frequency or constipation and has a prevalence of approximately 5%–12% among 5- to 10-year-olds. The appropriate functional exchange between the pontine micturition center, periaqueductal gray matter, and prefrontal cortex is important for proper micturition control. Several studies on pediatric cases observed a link between OAB and neuropsychiatric problems, such as anxiety, depression, and attention deficit, and treatment of these comorbidities improved patient symptoms. In this review, we present the pathophysiology of OAB, its associated conditions, and aspects related to updates in OAB treatment, and we propose a step-by-step treatment approach following this sequence: behavioral therapy, medical treatment, and invasive treatment. Although anticholinergic drugs are the mainstay of OAB medical treatment, beta-3 agonists and alpha-blockers are now recommended as a result of significant advancements in pharmacologic treatment in the last 10 years. Electrical stimulation techniques and botulinum toxin are also effective and can be used, especially in conventional treatment-refractory cases.
INTRODUCTION
Daytime lower urinary tract dysfunction (LUTD) is a common clinical disorder in the pediatric population, with a prevalence of 1%–20% [1–5], and comprises 40% of outpatient pediatric urologist clinic visits [6]. LUTD primarily results from dysfunction of the voiding phase, filling phase, or both and can vary in severity. Its 2 main types are overactive bladder (OAB) and dysfunctional voiding (DV).
In children, LUTD mainly results from delayed or incomplete maturation of the lower urinary tract complex (bladder sphincter). The pons is responsible for detrusor and sphincter coordination, while the cortex controls micturition reflex inhibition and voluntary micturition initiation [7]. Therefore, incomplete cortical maturation is proposed as the main reason for pediatric OAB, whereas failure of detrusor and sphincter coordination may be the primary reason for DV [8].
Among storage-phase dysfunctions, OAB is the most common and usually occurs in children between 5 and 7 years old, especially in boys [9]. According to the International Children’s Continence Society guidelines, OAB is defined as urinary urgency, usually accompanied by the frequency of urinary incontinence with or without urinary incontinence, and without any signs of urinary tract infection (UTI) or any other obvious pathology [10]. Some children habitually endure the urge to urinate by performing holding maneuvers, such as Vincent’s curtsy or squatting, leading to voiding postponement and sometimes bladder overdistention. Owing to a high-pressure bladder, recurrent UTI or vesicoureteral reflux may be common [7]. Another important risk factor for OAB that should be assessed separately is constipation. Toilet training involves adequate bowel and bladder storage and emptying at an appropriate place and time and is generally performed at 3–4 years of age. Toilet training problems, when accompanied by incontinence, can lead to low self-esteem in children [8]. In many instances, this can place undue stress on caregivers as a result of more frequent trips to the toilet.
PATHOPHYSIOLOGICAL FEATURES
Urgency
Urinary urgency is the cornerstone symptom for diagnosing OAB. When there is no urine in the bladder, the bladder pressure is almost zero; this rises to 5–10 cm H2O when 30–50 mL of urine accumulates in the bladder. An additional 200–300 mL of collected urine further raises the pressure slightly, and constant intravesical pressure is maintained due to the inherent tone of the bladder wall (compliance) [11]. Urinary contractions usually occur when the bladder is partially filled and disappear after a few seconds. As the bladder continues to fill, the voiding reflex frequency gradually increases, and detrusor contraction intensifies. Patients with OAB who experience a pathologically distinct urge to urinate resulting from a full bladder deviate from this norm and perceive micturition contractions as more intense than normal. This finding is thought to be related to changes in the threshold at which these contractions occur during partial bladder filling (Fig. 1).

Transition of bladder accommodation during filling. Bladder accommodation during filling is a passive process whereby bladder tone and intravesical pressure are maintained as urine accumulates. In patients with overactive bladder, a pathologic urge to urinate occurs in the initial maintenance phase owing to a decreased threshold.
The sensation of bladder fullness is transmitted through 2 different types of afferent fibers. Aδ-fibers are activated by low-intensity stimuli, such as bladder distention, and transmit normal sensations of bladder fullness. In contrast, C-fibers, which have a higher threshold, usually transmit pain sensations and play an important role in bladder diseases [12]. Not all patients with urinary urgency experience detrusor overactivity or sense bladder contractions, which are measured by 24-hour urodynamic studies (UDS). Therefore, detrusor overactivity is not a key defining feature of urinary urgency. However, Bael et al. [13] demonstrated that the use of UDS in children with OAB is limited.
Neural Factors
Normal development and immaturity
The pathophysiology of OAB has not been thoroughly studied and is believed to be multifactorial. One representative theory proposes that urinary urgency and related symptoms result from the immaturity of the neural centers controlling urination [14], which is supported by the fact that voiding ability improves with age. In the first few months of life, a child’s voiding pattern is characterized by an intermittent urinary flow [15]. During this period, micturition is controlled by the brainstem, similar to involuntary reflex voiding. At ages 1–3 years, voiding ability improves as the cortical inhibitory pathway, periaqueductal gray matter (PAG), anterior cingulate gyrus (ACG), and autonomic/somatic nervous system develop [6]. As the child matures, the prefrontal cortex (PFC) develops top-down control over more primitive afferent brain pathways, such as the limbic and paralimbic systems. Frontal lobe maturation occurs throughout childhood and adolescence until the mid-20s, which explains the gradual resolution of OAB as children age. Chung et al. [16] reported an age-dependent decrease in OAB prevalence from approximately 23% at 5 years to 12.2% at 13 years of age.
ACG hyperactivation and pontine center deactivation
Urgency is defined as the “intense desire” to urinate, suggesting that higher brain centers are involved in this process. Functional magnetic resonance imaging (MRI) and positron emission tomography revealed differences in brain lesions and activity between patients with normal LUT function and those with detrusor overactivity [17,18]. During the storage phase, the PAG, which is located in the midbrain, receives information from the medial PFC, ACG, thalamus/hypothalamus, and insula (Fig. 2) [17].

A preliminary brain neural circuit model during the storage phase. Ascending afferent synapses from the sacral spinal cord reach the PAG. These signals are relayed through the thalamus and hypothalamus to the dorsal ACG on one side and the right insula/lateral PFC on the other side. Subsequently, they move to the medial PFC, which is a decision-making area. When the decision to not urinate is made, chronic PAG inhibition through the long pathway is maintained; as a result, PMC is also suppressed and voiding does not occur. In children with urgency, ACG and insula activity increase while PFC activity decreases, thereby antagonizing chronic PAG inhibition. PAG, periaqueductal gray matter; ACG, anterior cingulate gyrus; PMC, pontine micturition center; PFC, prefrontal cortex.
An MRI-based study revealed that a small bladder volume is associated with decreased ACG activity, and higher bladder volumes correlate with significantly increased ACG activity [14]. Patients with urgency have increased ACG activity and decreased PFC activity, which leads to more frequent bladder repletion and, therefore, the urge to void.
Neuropsychiatric Problems
While the pathophysiology of urgency has not been fully elucidated, the relationship between LUTD, behavioral aspects, and constipation reaffirms the close association of the bladder–gut–brain axis [19]. In a longitudinal study, anxiety disorder was observed in children as young as 6 months old, and up to one-third of social anxiety persisted until the age of 15 [20]. Functional MRI data revealed thinning of PFC lesions in children with refractory OAB, while cortical thickening was observed as a compensatory mechanism for anxiety in children with improved symptoms. A relationship between irritable bowel syndrome (IBS) and OAB has also been observed, especially in IBS with chronic constipation. Similar to OAB, psychiatric comorbidities are prevalent in children with IBS [21]. Evidence of cortical thinning in the frontal lobe of patients with IBS supports the possibility of these comorbidities [22].
Children who delay urination may experience behavioral problems. Similar to learning and psychiatric disorders, such as panic, anxiety, attention deficit hyperactivity disorder, and oppositional defiant disorder [19,23], voiding postponement may be the reason for behavioral problems. Children with oppositional defiant disorder sometimes refuse to go to the toilet, which leads to functional fecal incontinence [24]. Although only a few studies on the neuroassessment of these patients have been published, PFC hypoactivation should be suspected [25,26].
Constipation
Several pelvic organs share an afferent innervation pathway (e.g., hypogastric nerve) that enables the crosstalk of neurons innervating other organs [27–29]. Children with OAB often experience urethral, vaginal, or penile discomfort, suggesting that pain is induced by a variety of anatomical lesions. Yoshimura et al. [30] reported that problems with the pelvic organs, such as the colon or uterus, affect urinary continence. Chronic colon distention due to constipation, which is a well-recognized cause of lower urinary tract symptoms (LUTS) in children, affects the bladder afferent signaling pathway, which prevents accurate recognition of the sensation of bladder fullness [31].
CLINICAL HISTORY AND PHYSICAL EXAMINATION
An in-depth medical history and physical examination should be performed when a child with OAB symptoms visits a hospital. Several papers have been published regarding the clinical workup of children with OAB [20,32]. When identifying symptoms suggestive of OAB, it is important to check for the presence of sacral dysraphism at the first examination. Excluding UTI from the diagnosis is also crucial and can be achieved by performing appropriate methods, such as urinalysis or, if necessary, a urine culture [20]. Checking for behaviors suggesting urgency (e.g., crossing of legs, running to the bathroom, grabbing the penis, rubbing the clitoris, squatting, or sitting on heels) is equally fundamental, and keeping a voiding diary may be necessary [33]. Abdominal palpation or radiographic assessment of constipation is paramount because parents are usually reluctant to admit that their child is constipated. In some children, a rectal diameter of approximately 3–4 cm suggests constipation, and this can be utilized instead of radiography [34]. Additionally, assessment of neuropsychiatric problems may be indicated for children, siblings, parents, and close relatives, as this can help clinicians immediately determine a child’s response to conventional treatment.
Over the past 20 years, several validated questionnaires have been developed to enable uniform history taking in children with LUTD. These questionnaires can help standardize clinical data reported in the literature and help clinicians measure outcomes. The followings are some of the validated questionnaires for evaluating LUTS in children:
(1) DV Symptom Score (first designed in 2000): This assessment tool is based on the American Urological Association Symptom Index [35].
(2) DV and Incontinence Scoring System: It consists of 14 questions, with a maximum score of 35+3 and a cutoff value of 9 points to determine abnormality [36].
(3) Pediatric Urinary Incontinence Quality of Life Score: Developed by Bower et al. [37], this tool has excellent internal validity, content validity, and test-retest reliability.
In addition to these, the Incontinence Symptom Index–Pediatric [38] and Child Behavior Checklist [39] can also be used. The Bristol stool chart [40] and Rome IV criteria [41] are references for evaluating bowel dysfunction. Because several LUTS may coexist, many children experience only partial symptom resolution with treatment. Therefore, the use of the abovementioned assessment tools may improve overall treatment benefits [32].
TREATMENT
Conservative Treatment
Urotherapy
Urotherapy is a nonsurgical, nonpharmacological approach (rehabilitation) that is considered a first-line treatment for OAB. Standard urotherapy begins with educating the child and family to explain and understand the child’s specific dysfunction. Motivation is an integral determinant of treatment success. A child who cannot anticipate the benefits of treating his or her dysfunction may not be ready to initiate treatment [42]. Timed voiding, approximately 6 times a day, can help relieve symptoms. For relatively large amounts of residual urine, double voiding is recommended. Fluid intake should be regular during the day and minimized before bedtime. In the presence of urinary urgency, DV, or abnormal defecation dynamics caused by problems with the pelvic floor or sphincter muscle relaxation during elimination, pelvic floor muscle training may be the next step [43]. For this, proper education should be provided to ensure an adequate voiding or defecation position where the feet are supported on a flat surface, with attention to the pelvic musculature to improve the coordination between bladder contraction and pelvic muscle relaxation [20]. A simple behavioral treatment can improve frequency and urgency rates in 50% of children [44]. For children unresponsive to simple urotherapy and those without cystourethrogram- or uroflowmetry-confirmed DV, pharmacotherapy is the treatment of choice.
Management of constipation
There are no standard evaluation and management protocols for constipation. Simple constipation may be treated by increasing hydration, adding high-sorbitol juices (e.g., prune, pear, or apple juice), or increasing the number of vegetables in a child’s normal diet. Avoiding excessive milk consumption is also helpful. Currently, there is no evidence supporting the treatment of constipation with pre/probiotic supplements [14]. If dietary interventions fail, polyethylene glycol (PEG), lactulose, or sorbitol may be administered. PEG may be introduced at an initial dose of 1.5 g/kg/day for 6 days, followed by 0.5–0.7 g/kg/day after 24 days.
Pelvic floor muscle retraining and biofeedback
Treatment-refractory OAB in children can be addressed using second-line approaches, such as biofeedback, transcutaneous electrical nerve stimulation, and botulinum toxin. In a study involving 24 children with standard urotherapy- and antimuscarinic therapy-resistant OAB, Pekbay et al. [45] reported that biofeedback-assisted pelvic floor muscle therapy provided symptomatic relief and increased functional bladder capacity.
Nonneurogenic voiding dysfunction in children is a dysmotility disorder related to external sphincter dysfunction. A recent systematic review revealed that biofeedback is effective in alleviating UTI symptoms or constipation. Moreover, biofeedback can improve postvoid residual and electromyography during voiding and has a longer-term effect [46].
Medications
General concept for pharmacologic therapy
Although stepwise therapy, such as behavioral treatment, is important, pharmacotherapy remains the mainstay of treatment. A well-balanced micturition cycle consists of coordinated sympathetic and parasympathetic activities; a reduced parasympathetic tone and an increased sympathetic tone may improve urgency. Muscarinic receptor blockers can be administered to regulate the parasympathetic tone; alpha or beta-agonists may be indicated for sympathetic tone regulation because, during the storage phase, the pontine micturition center is continuously activated and sends descending signals to the hypogastric nerve, which subsequently relaxes the detrusor muscle through beta-adrenoceptor (AR) activation and contracts the internal sphincter through alpha AR activation [10]. However, in practice, alpha agonists are not recommended for OAB because of their systemic effects (especially on vascular smooth muscles). In human bladder smooth muscles, 96% of sympathetic receptors are beta-3 receptors, which represent 97% of the total beta AR mRNA expression in the bladder [18]. Several studies have revealed that beta-3 AR stimulation is associated with increased micturition capacity without aggravating residual volume or contraction power. Targeting beta-3 ARs, therefore, increases the bladder storage volume without affecting the contraction amplitude [47–49].
Antimuscarinic agents
Antimuscarinics (also known as anticholinergics) are generally used as the first-line treatment in children who remain symptomatic after urotherapy. Currently, only 2 antimuscarinic agents have received formal approval for use in children (oxybutynin by the U.S. Food and Drug Administration [FDA] and European Medicines Agency [EMA]; tolterodine by the EMA). While several studies have reported significant results with fesoterodine, propiverine [50,51], solifenacin [52,53], and trospium [54], the administration of these drugs, which depends on age and national discipline, has not received official permission.
Although antimuscarinics are the mainstay of medical treatment, their use is associated with significant side effects, such as dry mouth, constipation, and blurred vision, especially at high doses [55]. Many antimuscarinics, including oxybutynin, cross the blood-brain barrier and bind to M2 receptors, negatively affecting cognitive functions in the central nervous system [56]. Adverse events include sedation, confusion, amnesia, and abnormal dreams, which are more common in children [57].
Oxybutynin
Oxybutynin is the most commonly used medication in the pediatric population, despite the absence of randomized clinical trials (RCTs) comparing it with a placebo. However, like adults, many children do not adequately respond to this drug [53]. Oxybutynin may be administered in either immediate-release (IR; 0.3–0.6 mg/kg recommended) or extended-release (ER; 5 mg or 10 mg, up to 20 mg/day) form. Various studies have demonstrated that ER formulations are superior to IR forms, with improved efficacy [58–60]. Unfortunately, they need to be swallowed intact. Intravesical instillation is an alternative formulation that allows for higher doses with fewer systemic side effects; however, the application scope is narrow because most children do not use intermittent catheters. Additionally, medical adherence to oxybutynin is not high. In their study, Van Arendonk et al. [61] reported that oxybutynin did not produce significant effects in any more than 20% of patients with daily incontinence; this is the main reason for discontinuing the medication and accounts for approximately 10% of cases [62]. Chapple et al. [63] reported a discontinuation rate with oxybutynin of approximately 32%.
Tolterodine
Tolterodine is also available in 2 formulas (IR and ER), and several studies have reported good efficacy and tolerability in children [64,65]. An RCT confirmed the safety, but not the efficacy, of tolterodine [66]; however, Medhi et al. [67] showed that tolterodine was as effective as oxybutynin and was associated with fewer side effects.
Fesoterodine
Fesoterodine is a long-acting antimuscarinic that can be administered in 4- or 8-mg doses. It is recommended for children weighing >25 kg and is well tolerated by children and adolescents [68].
Solifenacin
Solifenacin is a slow-acting antimuscarinic that is available at 5 or 10 mg. A recent open-label safety extension study by Newgreen et al. [69] confirmed the efficacy of solifenacin, with constipation (12%) and electrocardiogram QT prolongation (9%) as side effects. The same authors also published a phase 3 RCT demonstrating that once-daily oral suspension of solifenacin was superior to a placebo in improving voiding volume parameters [70]. As an extension of this study, the study by Blais et al. [71] revealed that solifenacin was effective and well tolerated in children.
Propiverine
An RCT published in 2009 showed that propiverine significantly improved urinary frequency, urinary incontinence episodes, and mean voided volume [72]. Kim et al. [51] reported an overall response rate of 86.8% in a retrospective review involving 68 children. Moreover, a multicenter observational cohort study demonstrated that propiverine was as effective as oxybutynin, and the tolerability profile of propiverine was more favorable [73].
Beta-3 AR agonists
Beta-3 AR agonists, which were approved for their favorable tolerability profile in pivotal phase 2 and 3 studies, are currently one of the main treatment options for adult patients with OAB. Their use in children with neurogenic detrusor overactivity has only recently been approved by the FDA [50,63,74]. Beta-3 AR agonists increase bladder capacity without changing the bladder pressure or postvoid residual volume by promoting the relaxation of bladder smooth muscles [14]. Compared with antimuscarinics, beta-3 AR agonists show adequate efficacy and have fewer adverse effects (dry mouth 2.0%, constipation 1.6%, headache 2.0%) [74].
The generally accepted mechanism of smooth muscle relaxation comprises downstream adenyl cyclase activation and subsequent cyclic adenosine monophosphate (cAMP) production, followed by Rho-kinase pathway inhibition [75,76]. Additionally, increased cAMP levels lead to protein kinase A activation, which activates large-conductance, calcium-dependent K+ (BK) channels. These activated BK channels hyperpolarize bladder smooth muscles, which increases detrusor stability [77]. Despite the paucity of literature, promising results have recently been reported. In their study, Blais et al. [71] demonstrated that mirabegron is safe and effective in children (median age, 10.1 years) with idiopathic, antimuscarinic-refractory OAB. The results showed that the median bladder capacity (150 → 200 mL), continence rate (89.6%), and quality of life significantly improved without noticeable side effects (P < 0.001).
Having fewer treatment options for pediatric OAB creates significant problems, especially in treating refractory OAB because the next level of therapy is frequently more invasive. In a prospective, off-label study, Morin et al. [78] employed a combination of mirabegron and a standard, maximized antimuscarinic for a mean of 16 months to treat 35 children (median age, 10.3 years) who had an inadequate response or refractory side effects during a 6-month trial of escalating doses of oxybutynin-ER, solifenacin, or fesoterodine. Continence improved in all children, and the median bladder capacity increased from 50% to 74% of that expected for age; 12 remained completely dry, and 2 were withdrawn owing to side effects.
Including the above 2 studies, Kim et al. [79] recently reported the first systematic review (3 prospective and 5 retrospective studies) suggesting the therapeutic role of beta-3 agonists in children: beta-3 agonists significantly improve urodynamic parameters and self-reported outcomes, such as incontinence. After treatment, the pooled effect estimates for maximum cystometric capacity had a mean difference of +99 mL (95% confidence interval, 74.72–122.96).
Invasive Treatment
Botulinum toxin
Clinical trials involving intravesical injections of botulinum toxin A (BoNTA) in children with OAB are currently underway. Despite its off-label use, BoNTA is an alternative for anticholinergic-intolerant patients. BoNTA blocks acetylcholine release at the parasympathetic presynaptic neuromuscular junction, leading to decreased regional muscle contractility and injection site atrophy. Subsequent chemical denervation is a reversible reaction, and inactivation and removal occur over time. The clinical effect appears after approximately 5–7 days, and the maximum effect appears after 4–6 weeks [80]. The paralytic effect varies depending on the muscle type at the injection site; however, it is known that it usually lasts for 3–9 months with no apparent tachyphylaxis [81]. The major disadvantage of BoNTA injections is the need for re-treatment due to temporary chemical denervation, which occurs because synaptic respouting occurs after approximately 6 months. According to a preliminary study, repeated BoNTA injections are safe for children and do not cause additional bladder wall fibrosis [82]. In another histological study, no ultrastructural changes were noted in the bladder muscles after Botox injection [83].
Other studies used varying Botox doses, ranging from 50 to 200 units (up to 200–300 units is commonly used for neurogenic OAB) [84–86]. Botox should be used in children over 3 years old at a dose of 5–10 units/kg of body weight [87]. Furthermore, because the effect of Botox is cumulative when treating a child with spasticity, precautions should be taken to determine a history of BoNTA administration at another site within the same period [9]. The full clinical response, defined by complete continence in pediatric patients, was 44%–95% in 2 level 3 studies [84,88]. Moreover, urodynamic improvements (38%–70%) were also reported in 2 laboratory studies [75,76].
Neuromodulation
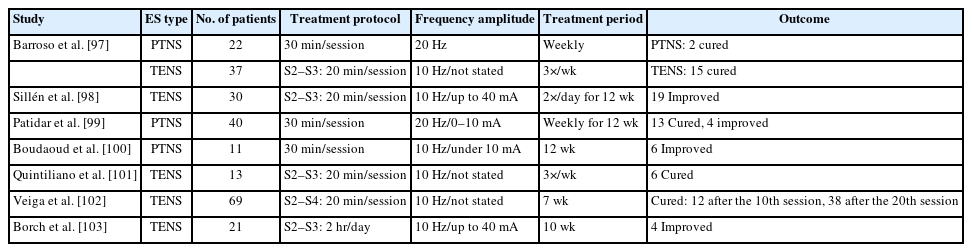
Over the last 2 decades, neuromodulation has been employed to treat children with nonneurogenic LUTD (Table 1), and several studies have reported that it can significantly improve bladder volume, decrease urgency severity, improve continence, and decrease UTI incidence [89–91]. Urodynamic parameters, including bladder compliance, episodes of detrusor overactivity, and volume at first detrusor contraction, also significantly improved [92].
Neuromodulation delivers electrical stimulation that changes existing neural transmission patterns and modulates detrusor activity. It also rebalances excitatory and inhibitory information to put the neural drive into a more neutral state. Although the exact mechanism of action remains unclear, data from many studies reveal that this approach is effective. A meta-analysis has reported that neuromodulation may lead to better partial improvement in children with nonneurogenic OAB; however, a complete response is not guaranteed [93]. The EAU guidelines recommend various neuromodulation modalities only in cases refractory to standard therapy [94]. Despite an initial successful response, a high recurrence rate that warrants long-term follow-up has been reported [95]. Additionally, many children may present with other forms of LUTD as adults [96].
CONCLUSION
OAB is characterized by the inability to properly suppress the urge to urinate. It is a bothersome syndrome that usually begins during childhood and continues into adulthood. Evidence suggests that this condition is primarily related to abnormalities in bladder sensation; therefore, adequate knowledge of neurophysiology may help in OAB management. More studies analyzing the central nervous system as the primary site of voiding dysfunction and the appropriate site for targeted treatment are required. Furthermore, continued research on functional neuroimaging is vital if greater advances are to be made in this field.
Notes
Funding/Grant Support
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2021R1G1A1014047).
Conflict of Interest
MMO, a member of the Editorial Board of International Neurourology Journal, is the corresponding author of this article. However, she played no role whatsoever in the editorial evaluation of this article or the decision to publish it. No potential conflict of interest relevant to this article was reported.