Advancing Patient Care: Innovative Use of Near-Infrared Spectroscopy for Monitoring Urine Volume in Neurogenic Bladder
Article information
Abstract
Purpose
Current guidelines recommend clean intermittent catheterization (CIC) at regular time intervals for patients with spinal cord injuries; however, many patients experience difficulties. Performing time-based CIC outside the home is a significant burden for patients. In this study, we aimed to overcome the limitations of the current guidelines by developing a digital device to monitor bladder urine volume in real-time.
Methods
The optode sensor is a near-infrared spectroscopy (NIRS)-based wearable device intended to be attached to the skin of the lower abdomen where the bladder is located. The sensor’s primary function is to detect changes in urine volume within the bladder. An in vitro study was conducted using a bladder phantom that mimicked the optical properties of the lower abdomen. To validate the data in the human body at the proof-of-concept level, one volunteer attached the device to the lower abdomen to measure the light intensity between the first voiding and immediately before the second voiding.
Results
The degree of attenuation at the maximum test volume was equivalent across experiments, and the optode sensor with multiplex measurements demonstrated robust performance for patient diversity. Moreover, the symmetric feature of the matrix was deemed a potential parameter for identifying the accuracy of sensor localization in a deep-learning model. The validated feasibility of the sensor showed almost the same results as an ultrasound scanner, which is routinely used in the clinical field.
Conclusions
The optode sensor of the NIRS-based wearable device can measure the urine volume in the bladder in real-time.
INTRODUCTION
Neurogenic bladder refers to bladder dysfunction resulting from damage to the central or peripheral nervous system [1]. It is characterized by various symptoms, including urinary incontinence, which can result in involuntary urine leakage and urinary retention, which is the inability to completely empty the bladder. Additionally, patients can exhibit bladder spasticity, which is an uncontrolled, involuntary contraction of the bladder muscle that can lead to urgency and frequent urination, as well as bladder flaccidity, which is a lack of muscle tone in the bladder that can result in difficulty in initiating urination. Patients may also experience recurrent urinary tract infections [2]. It is important to note that these signs and symptoms can vary in severity depending on the level and extent of spinal cord injury, brain and the patient’s response to treatment [1,3,4].
It is important to note that the management of neurogenic bladder should be individualized based on the patient’s specific symptoms, functional abilities, and care goals. Clean intermittent catheterization (CIC), a current management option, is the recommended first-line therapy for neurogenic bladders who are unable to completely and/or effectively empty their bladders [5,6]. CIC is employed to empty urine from the bladder at regular intervals throughout the day. The procedure involves inserting a sterile lubricated catheter through the urethra into the bladder to drain the urine, which is typically left in place for a few seconds to allow complete emptying of the bladder before removal. CIC is usually performed several times per day depending on the patient’s individual needs and bladder function.
According to the recent guidelines for neurogenic bladder management by the European Association of Urology (EAU), American Urological Association (AUA), and Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction (SUFU), the recommended CIC for patients with spinal cord injury is 4–6 times per day [7-9]. However, these guidelines are severely limited by the lack of patient perspectives. The frequency and timing of CIC may need to be adjusted according to individual patient needs and bladder function. Due to recently released guidelines on neurogenic bladder management from the EAU, AUA, and SUFU, patients with spinal cord injury are reluctant to go out, sometimes give up on social activities, and feel less comfortable working outdoors. Patients constantly feel stressed about performing catheterization on time. However, this is not the best solution.
When patients undergo CIC, the amount of urine in the CIC can vary significantly, ranging anywhere from 100 to 1,000 mL. Patients who cannot sense their urge to void must monitor their bladder urine volume. However, eating meals or drinking water each day can greatly influence biorhythms. Therefore, the question arises whether the self-catheterization interval should always be the same. Patients try to determine their own CIC frequency, but it is difficult to perform CIC on time, and many simply delay or give up. Frequent excessive bladder filling can lead to urinary tract infection and damage to upper urinary tract function.
To overcome these limitations, near-infrared spectroscopy (NIRS)—a noninvasive and cost-effective modality for quantifying the concentrations of tissue oxyhemoglobin, deoxyhemoglobin, water, and lipids—has been used to monitor neurogenic bladders. Considering portable technology and quantification of physiological information, spatially resolved spectroscopy, a NIRS technique, represents the most appropriate methodology for wearable digital healthcare devices [10-12].
NIRS has been proposed for monitoring bladder urine volume by measuring the absorption and scattering of near-infrared (NIR) light in tissues using an optode sensor equipped with a NIR light source and an optical detector array at several distances [13,14]. As urine accumulates within the bladder, changes occur in the thickness and composition of the tissue between the optode sensor and the bladder, which, in turn, causes variations in the amount of light absorbed or scattered by the tissue. These changes in light absorption or scattering can be detected by an optode sensor and used to estimate the bladder urine volume.
NIRS has the advantages of being noninvasive, portable, and easy to use, making it a promising technology for bladder urine volume monitoring in digital healthcare applications, particularly in situations where frequent and noninvasive monitoring is required.
This study demonstrates that NIRS can be a reliable and accurate method for real-time monitoring of bladder urine volume with comparable accuracy to more invasive and temporary checking methods, such as catheterization or ultrasound.
MATERIALS AND METHODS
Optode Sensor
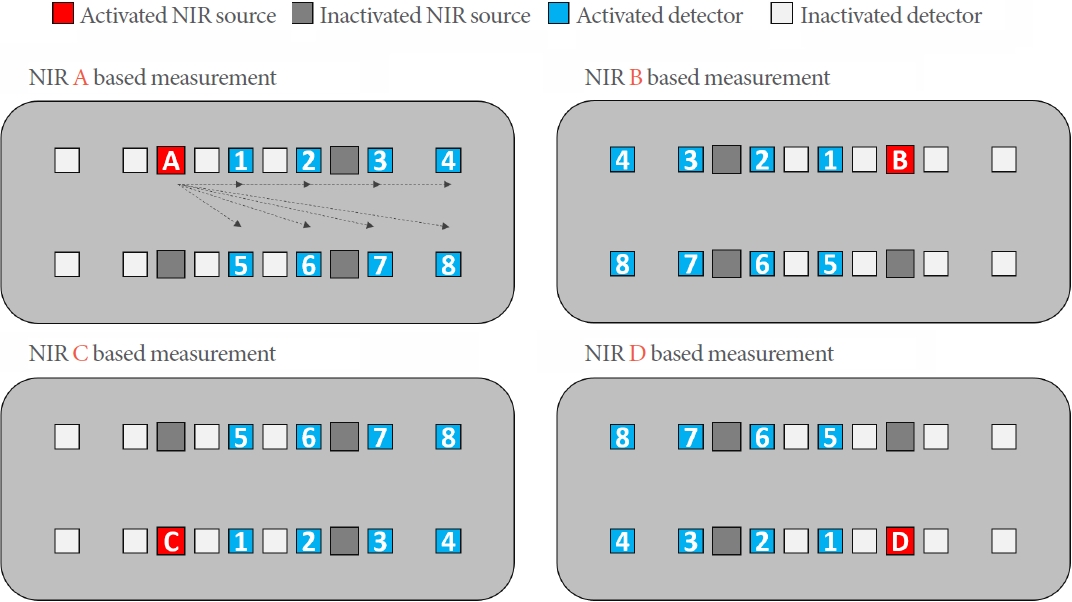
The optode sensor is a NIRS-based wearable device intended to be attached to the skin of the lower abdomen, where the bladder is located. It consists of wireless hardware comprising 4 NIR light sources and 16 detectors. Fig. 1 shows the layout of the optode sensor. The NIR light from each source is detected using 8 detectors. The source-detector separations (SDS) of the optode sensor are 10.0–40.0 mm horizontally and 18.0–42.7 mm diagonally. The left-side sources were paired with the right-side detectors, and vice versa for the right-side sources. The optode sensor measured 106 mm×68 mm×15 mm and weighed less than 50 g. It can be controlled wirelessly using a personal computer (PC) or mobile phone.
Fabrication of Bladder Phantom
A bladder phantom mimicking the optical properties of the lower abdomen was developed (Fig. 2). The bladder phantom is a 3-dimensional (3D)-printed container box consisting of a silicone-based wall on one side with a syringe-connected latex balloon inside. The silicone-based wall, which reflects the optical properties of the lower abdomen layer (absorption coefficient, μa; 0.01 mm-1mL-1 at 660 nm and reduced scattering coefficient, μ’s; 1.2 mm-1g-1 at 660 nm) [15], was constructed using a silicone base (P-4, Silicones, Inc., High Point, NC USA), a silicone activator (P-4, Silicones, Inc.), nigrosine (μa; 0.006 mm-1mL-1 at 660 nm; Sigma-Aldrich, Burlington, MA, USA), and titanium (IV) oxide anatase (TiO2, μ’s; 1.71 mm-1g-1 at 660 nm, Sigma-Aldrich). The latex balloon was located 3 cm away from the silicone base wall and attached to the silicone-based wall by expansion from water filling through a syringe. The 3D-printed container box was filled with 20% Intralipid.
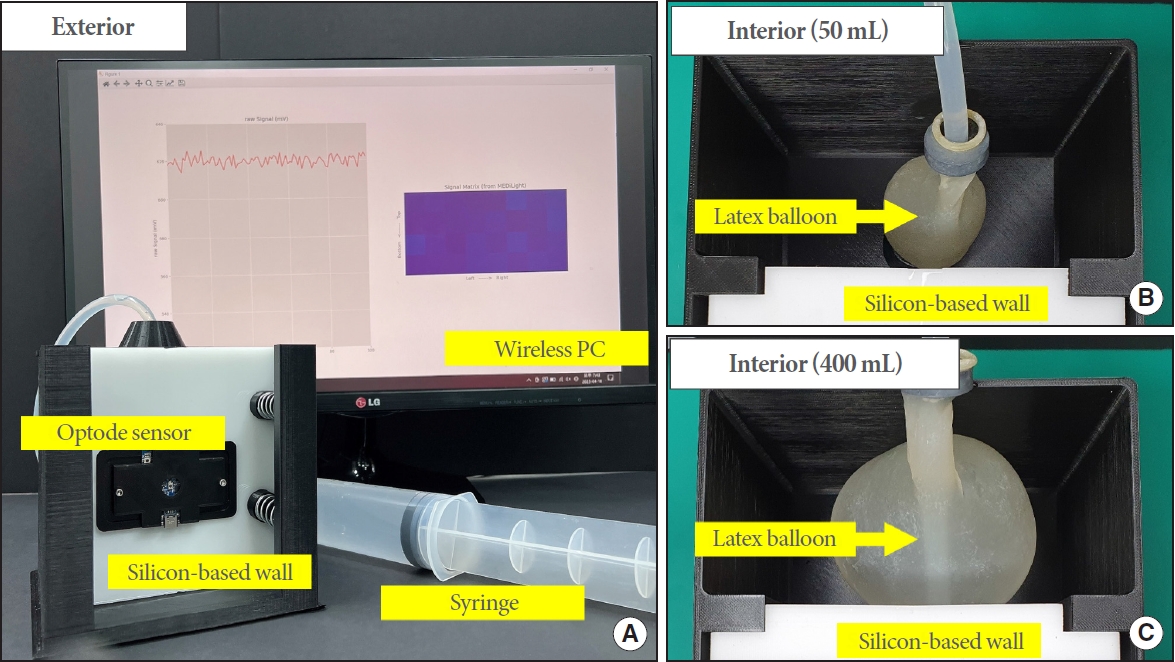
Bladder phantom-based experiment. (A) Bladder phantom setting. The bladder phantom is a container with one silicon-based wall. The top of the phantom is connected to a syringe for water filling and emptying of the latex balloon that is located inside. The optode sensor is attached to the silicon-based wall and near-infrared spectroscopy are irradiated into the phantom. The measurement data is collected wirelessly on a personal computer. (B, C) Interior of the bladder phantom. The latex balloon that is connected to the syringe is located inside the phantom and expands according to the water filling. The intralipid filled in the phantom was removed to show the change of the latex balloon.
Experiment Set-up for an In Vitro Phantom Study
An in vitro study was conducted using the bladder phantom composed of a silicon-based wall with a thickness of 2.0 cm. The latex balloon was connected to a syringe containing 400 mL of water. The interior of each phantom was filled with milk. The optode sensor was attached to the silicone-based wall at a position where NIR light could pass through the center of the latex balloon. The sensor was controlled wirelessly and data were collected on a PC. A single measurement cycle collected 32 pairs (4 NIR sources×8 detectors) of data and required approximately 1 second.
After starting the measurement, the latex balloon was gradually filled with water from 50 to 400 mL and then gradually removed manually to return it to 50 mL again. The data were collected after 5 measurement cycles to stabilize the optode sensor.
In Vivo Study in the Proof-of-Concept: Preliminary Experiment for Clinical Feasibility
The feasibility of the clinical utility of the optode sensor was validated using a volunteer at the proof-of-concept level. AK, the urologist and principal investigator of the study, voluntarily performed self-catheterization using an 18F. Foley catheter. First, the bladder was completely emptied and confirmed to be 0 mL using an ultrasound scanner. Next, the optode sensor was attached to the lower abdomen, and another staff member filled the bladder with normal saline through a catheter to measure real-time bladder urine volume. Light intensity was measured between the first and second voiding. Finally, light intensity attenuation was analyzed by comparing the light intensity of the first measurement after the first voiding.
RESULTS
Bladder Volume Measurement
The main objective of the optode sensor is to detect changes in bladder urine volume. The feasibility of the optode sensor for this objective was evaluated by monitoring the changes in light intensity during water filling and emptying in the bladder phantom.
Data were collected for each pair of NIR source detectors and the relative light intensity was analyzed for each pair. The relative light intensity was calculated using the following formula, which takes the ratio of each measurement (I) to the first collected data (I0):
Fig. 3 shows the light intensity curves according to the water filling and emptying. The x-axis represents the measurement cycle, while the y-axis represents the mean relative light intensity of 32 pairs of data. As expected, the relative light intensity was attenuated by water filling and recovered by water emptying. This pattern was conserved in 3 experiments using 3 individual optode sensors (Fig. 3B). In particular, the degrees of attenuation at the maximum test volume (400 mL) were equivalent across the 3 experiments, indicating the reproducible performance of the optode sensor.
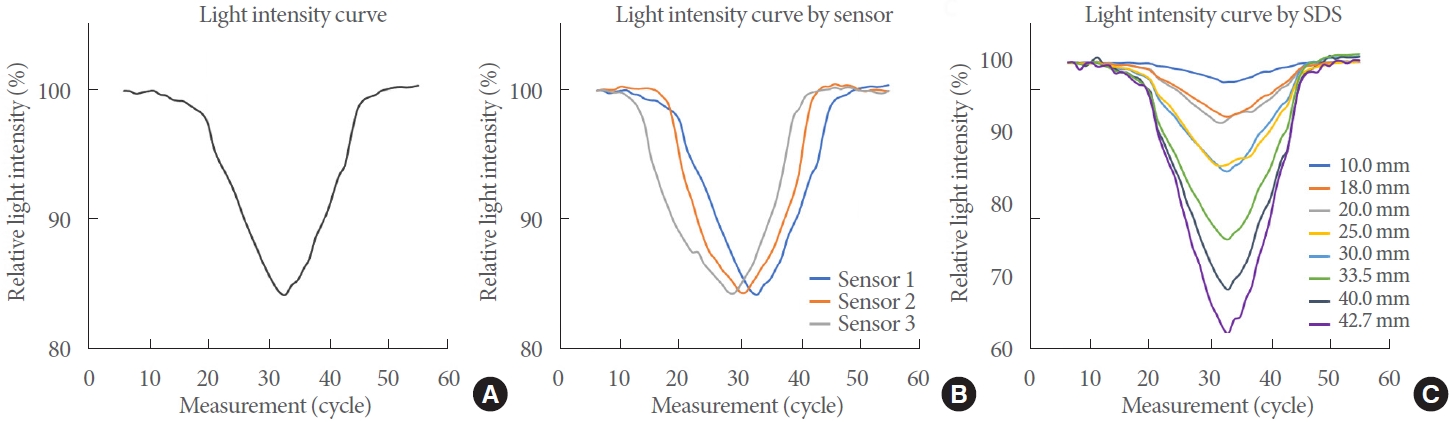
Feasibility of the optode sensor. The light intensity curve shows the relative light intensity (y-axis) to the continuous measurement cycle (x-axis). (A) The light intensity decreased during water filling and increased during water emptying. (B) Reproducibility of the optode sensor. The experiment was repeated 3 times using 3 individual optode sensors. (C) Spatial analysis. The width of attenuation differed across the source-detector separations (SDS).
When we separately analyzed the light intensity by SDS, the pattern of light intensity attenuation was similar. However, as shown in Fig. 3A and B, the width differed across the SDS (Fig. 3C); specifically, the greater the SDS, the faster the attenuation and the deeper the width. This reflects the structure of the bladder phantom, such as the thickness of the silicon-based wall and the distance of the latex balloon from the wall, and implies that the optode sensor with multiplex measurements has robust performance for patient diversity.
Physiological Quantification of the Optode Sensor
Physical features (e.g., the bladder structure and abdomen thickness) vary among patients. Patients with neurogenic bladder are considered to depend on the disease progression. As these features can affect the optical properties of biological tissues, physiological quantification using the NIRS technique can be based on the various features of the patient.
We adopted narrowband diffuse reflectance spectroscopy (nb-DRS) methodology [16] to assess the capability of our optode sensor to monitor physiological changes. Fig. 4 shows the water content matrix from the nb-DRS, which was produced by estimating the water content fraction for each pair of NIR sources and detectors and rearranging them based on the location of the optical materials and optical path depth according to the SDS.
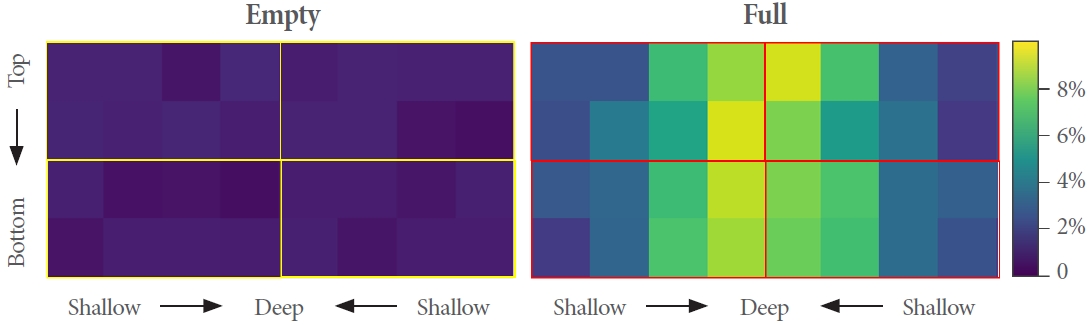
Water content estimation. The matrix is a map of the physiological change in water content in the optical path of each pair of the near-infrared (NIR) sources-detectors. Each area indicates a pair. The top two and bottom rows are analyses of the NIR sources located at the top and bottom, respectively. Horizontally closer to deep, the analysis of further source-detector separations detector.
By comparison with an empty matrix, we were able to present the spatial distribution of the water content in the bladder phantom. Most importantly, the symmetry feature of the matrix can be a potential parameter for identifying the accuracy of sensor localization in deep-learning models.
Preliminary Study for Clinical Feasibility
The feasibility of the clinical utility of the optode sensor was validated using a volunteer at the proof-of-concept level. Light intensity was measured between the first and second voiding, and light intensity attenuation was analyzed by comparing the light intensity of the first measurement after the first voiding.
Fig. 5 shows the light intensity curve between post-first and pre-second voiding. The light intensity attenuated continuously over time and was approximately 65% of that at the starting point when the subject started the second void. The subject voided after this, implying that the optode sensor could be used to monitor changes in bladder urine volume.
DISCUSSION
Advances in medicine have been made through extensive research and innovation. This innovation arises from unconventional thinking, efforts, and challenges on the part of individuals who are not satisfied with the current state of technology and research. Wearable devices are being developed to overcome diseases for purposes such as disease monitoring, screening, detection, and prediction, and the pace of development is astonishingly fast [17]. Wearable devices provide innovative solutions to diagnose and treat diseases by providing functions that replace or surpass those of human sensory organs [18]. So far, the development of wearable devices is a bright achievement in the combination of medicine and technology. When facing challenges in clinical practice that cannot be overcome by the current guidelines, it is crucial to accept and follow them. However, to surpass these limitations, innovative thinking and daring challenges are also necessary.
The bladder-monitoring patch introduced here was developed to address the issue of bladder sensation loss in patients with spinal cord or brain injuries. Currently, ultrasound scanners are used in clinical practice to measure residual urine volume; however, these devices are expensive, heavy, and require medical professionals to operate them. Moreover, they are limited to only measuring the urine volume inside the bladder at the specific time of measurement and are not wearable. Additionally, ultrasound scanners must maintain contact with the skin using a gel in order to work. In contrast, the bladder-monitoring patch introduced in this study is a wearable device that can remain constantly attached to the body, thus enabling patients with spinal cord injuries to know their bladder urine volume at any time. As a result, patients can reduce unnecessary self-catheterization, which can significantly reduce complications arising from bladder distension. To the best of our knowledge, this is the first study to develop an NIRS-based wearable bladder-monitoring device for patients with neurogenic bladders.
Furthermore, the novel bladder-monitoring patch greatly assists patients in engaging in external activities, such as work or travel, promoting their economic participation after rehabilitation. This device can also be applied to patients who experience decreased bladder sensation or those who do not report it owing to conditions such as Alzheimer disease, stroke Parkinson disease or diabetes. This will help decrease issues such as recurrent urinary tract infection and the resulting antibiotic resistance problems, urinary stones, vesicoureteral reflux, and pyelonephritis, thereby promoting upper urinary tract safety.
Future works should include optimizations that allow for enhanced wearable features of digital healthcare devices. In this study, we evaluated the performance of an optode sensor by testing it on an in vitro phantom, which is a biomimetic specimen, and by conducting a preliminary case study. However, these parameters may not apply to all cases. For example, motion artifacts, sensor localization, and additional clinical trials should be considered when designing wearable medical devices. Our plan is to develop a deep-learning model that addresses the aforementioned issues and validate its performance through clinical trials involving patients with neurogenic bladders. Accordingly, we expect that our optode sensor can serve as a basis for new guidelines for patients with neurogenic bladders.
In conclusion, the bladder-monitoring patch introduced in this study was an attempt to overcome the limitations of the current guidelines. Using this device, patients with neurogenic bladders, including those with spinal cord injury, can monitor their bladder urine volume in real-time. This reduces unnecessary episodes of self-catheterization, allowing patients to engage in external activities such as work or travel. We hope that the convergence of cutting-edge science and medicine will provide patients with a better quality of life.
Notes
Grant/Fund Support
This research was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Science and Technology and the Ministry of Education, as well as the Korea Technology Information Promotion Agency (TIPA) funded by the Ministry of SMEs and Startups (NRF-2022R1I1A3073688, 2023K1A4A3A02057280, S3321192).
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTION STATEMENT
· Conceptualization: BIK, AK, SK
· Data curation: BIK, AK, SK
· Formal analysis: BIK, SK
· Funding acquisition: SK
· Methodology: BIK, AK, SK
· Project administration: BIK, AK, SK
· Visualization: BIK, AK, SK
· Writing - original draft: BIK, AK, SK
· Writing - review & editing: AK, SK
Acknowledgements
We greatly appreciate Dr. Aram Kim, who is the project investigator, for happily volunteering to validate the feasibility of our optode sensor for the preliminary study.