Role of Pelvic Ischemia in Human Lower Urinary Tract Symptoms and Sexual Function Among Patients With Common Iliac Artery Obstruction Undergoing Revascularization Surgery
Article information
Abstract
Purpose
In this case-control study, we explored the relationships among pelvic ischemia, lower urinary tract symptoms (LUTS), and sexual function in patients with common iliac artery steno-occlusive disease, along with the potential therapeutic role of revascularization.
Methods
We recruited 33 men diagnosed with radiologically documented common iliac artery stenosis (>80%) who underwent endovascular revascularization, and 33 healthy controls. Five patients had obstruction of the abdominal aorta (Leriche syndrome). The International Prostate Symptom Score (IPSS), Overactive Bladder Questionnaire, and International Index of Erectile Function were used to evaluate LUTS and erectile function. Medical history, anthropometrics, urinalysis, and blood tests, including levels of serum prostate-specific antigen, urea, creatinine, triglycerides, cholesterol, low-density lipoprotein, high-density lipoprotein, and hemoglobin A1c, were recorded. Uroflow (maximum flow, average flow, voided volume, and voiding time) and ultrasound parameters (prostate volume and postvoid residual [PVR]) were also measured. Patients with moderate-to-severe LUTS (IPSS>7) underwent complete urodynamic investigation. Patients were examined at baseline and 6 months postoperatively.
Results
Patients exhibited poorer total IPSS (P<0.001), storage (P=0.001) and voiding symptom (P<0.001) subscores, as well as worse OAB-bother (P=0.015), OAB-sleep (P<0.001), OAB-coping (P<0.001), and OAB-total (P<0.001) scores than control participants. Additionally, erectile function (P=0.002), sexual desire (P<0.001), and satisfaction from intercourse (P=0.016) deteriorated in the patient group. Six months postoperatively, significant improvements were observed in erectile function (P=0.008), orgasm (P=0.021), and desire (P=0.014). Similarly, PVR significantly improved (P=0.012), while fewer patients experienced increased bladder sensation (P=0.035) and detrusor overactivity (P=0.035) upon postoperative urodynamic study. No significant differences were found between patients with bilateral and unilateral obstruction or between either of those groups and Leriche syndrome patients.
Conclusions
Patients with steno-occlusive disease of the common iliac artery experienced more severe LUTS and sexual dysfunction than healthy controls. Endovascular revascularization alleviated LUTS in patients with moderate-to-severe symptoms and improved bladder and erectile function.
INTRODUCTION
The role of atherogenesis and consequent pelvic ischemia in the pathogenesis of lower urinary tract symptoms (LUTS) and erectile dysfunction (ED) is increasingly being investigated [1]. Results from animal studies involving experimental iliac artery obstruction support the association between vascular obstruction and LUTS. Early-stage pelvic ischemia has been linked to bladder overactivity, while bladder underactivity is observed in later phases [2-4]. Moreover, extended periods of ischemia result in structural and functional changes in the urinary bladder musculature and endothelium, as well as alterations in bladder innervation and microvasculature [5].
Pelvic atheromatosis, primarily found in the iliac artery bifurcation and abdominal aorta, is prevalent among the older population and results in reduced blood flow to the urinary bladder and genitalia [5]. Moreover, aging and comorbidities promote morphological and functional changes in the urinary bladder, as well as alterations in its innervation and vasculature. Specifically, aging induces oxidative stress by disrupting the production of vasoconstrictor and vasodilator factors and affecting angiogenesis [4]. Additionally, metabolic syndrome and its components, particularly diabetes mellitus (DM), have been found to be associated with LUTS, while significant aorto-iliac disease has been putatively linked to the presence of LUTS [6,7]. Furthermore, internal pudendal artery stenosis has been demonstrated to be associated with ED [8]. Finally, several clinical studies support the relationships of cardiovascular risk factors, sexual dysfunction, and aging with LUTS [9].
The impact of high-grade common iliac artery stenosis and obstruction, as well as its treatment, on LUTS in humans has not been clearly established; however, it is important to consider that peripheral vascular disease in humans is a chronic process that allows revascularization and adaptation of the urinary bladder to hypoxia. Consequently, findings from animal models may not be readily applicable to humans.
To assess the impact of common iliac artery steno-occlusive disease and the potential therapeutic benefits of endovascular revascularization on LUTS and sexual function, we conducted a study examining patients with unilateral or bilateral common iliac artery critical stenosis (>80%) and obstruction, both before and after revascularization surgery, and compared them to healthy individuals. In addition to utilizing self-reported questionnaires to evaluate bladder and sexual function, this study is the first to incorporate urodynamics for an objective assessment of bladder function pre- and postrevascularization.
MATERIALS AND METHODS
Summary of Methods
We recruited patients with intermittent claudication (Fontaine classification IIb-III) resulting from either unilateral or bilateral critical iliac artery stenosis, who were scheduled for elective surgery by a vascular surgeon. After documenting each patient’s medical history, we conducted a full urological examination, including International Prostate Symptom Score (IPSS) and International Index of Erectile Function (IIEF) assessment, blood testing, and transabdominal ultrasound. This baseline examination was repeated 6 months after revascularization surgery. Patients with an IPSS greater than 7 also underwent complete urodynamic evaluation. Additionally, we utilized a control group of age- and sex-matched individuals (IPSS <8 and no history of urological surgery) for baseline comparisons.
Detailed Description of Methods
Our study employed a cohort design with a case-control approach. We enrolled adult patients presenting with unilateral or bilateral common iliac artery stenosis (>80%) or complete obstruction, who were admitted to the vascular surgery department of a public teaching hospital between April 2017 and December 2019 for endovascular revascularization surgery. The control group consisted of healthy individuals from the hospital personnel who were age- and sex-matched, reported no history of LUTS, and scored below 8 on the IPSS.
All enrolled patients were diagnosed with high-grade common iliac artery stenosis (>80%) or complete obstruction, as determined by magnetic resonance or computed tomography angiography, and experienced intermittent claudication (Fontaine classification IIb-III). Participants were scheduled for endovascular revascularization surgery. Based on the imaging results, patients had either unilateral or bilateral common iliac artery obstruction. Those with Leriche syndrome, characterized by obstruction of the lower abdominal aorta and common iliac arteries, were also included. The exclusion criteria consisted of a history of lower urinary tract surgery, urological cancer, bladder stones, neurogenic lower urinary tract dysfunction, use of medications affecting micturition within the 6 months prior to enrollment, and unsuccessful surgery or the need for additional surgical treatment.
All patients were examined at baseline and at 6 months postrevascularization. All participants completed the IPSS and Overactive Bladder Questionnaire (OAB-q), while the IIEF was used to evaluate sexual function. In accordance with the routine evaluation of LUTS, participants underwent uroflowmetry, which measured maximum flow (Qmax), average flow (Qave), voided volume, and voiding time. Additionally, transabdominal ultrasound was performed to measure prostate volume and postvoid residual (PVR) in all patients and control participants. Patients with an IPSS greater than 7 (n=8) also underwent urodynamic study (UDS) before and after the intervention. During those evaluations, maximum cystometric capacity, maximum detrusor pressure (Pdetmax) during filling, Pdetmax during voiding, PVR, bladder sensation, and the presence of detrusor overactivity (DO) were recorded, in line with routine assessment. The Bladder Contractility Index (BCI) and Bladder outlet obstruction Index (BOOI) were also calculated.
Age, anthropometric measurements (height, weight, and waist circumference), social factors (smoking, alcohol consumption, and weekly hours of exercise), and medical history (DM and hypertension) were used as independent variables. Body mass index (BMI) was also calculated. Additionally, baseline urinalysis and blood tests, including serum prostate-specific antigen, urea, and creatinine levels, were performed to exclude infection, cancer, and renal injury. Triglycerides, cholesterol, low-density lipoprotein, high-density lipoprotein (HDL), and hemoglobin A1c were routinely measured to assess the atherogenic profile and its association with LUTS and sexual function.
Study Questionnaires
International Prostate Symptom Score
LUTS were categorized as asymptomatic (IPSS 0–7), moderate (8–19), or severe (20–35). A score of 8 or higher indicated the presence of clinically significant LUTS. Additionally, IPSS voiding and storage scores were estimated [10].
International Index of Erectile Function
The validated Greek version of the IIEF was utilized to evaluate male sexual function. Questions 1–5 and 15 focused on erectile function, items 9–10 on orgasm, items 11–12 on sexual desire, items 6–8 on satisfaction from intercourse, and items 13–14 on overall satisfaction. Scores ranged from 0 to 5 on a Likert scale. The extensive use of this questionnaire enables comparisons among various populations [11,12].
Overactive Bladder Questionnaire
The validated Greek version of this 33-item questionnaire [13] was utilized to evaluate the presence and severity of OAB symptoms, as well as their impact on health-related quality of life (HRQoL). In addition to the total score, questions 9, 11, 16, 21, 22, 26, 32, and 33 assess patient coping strategies, while items 12, 13, 14, 19, 23, 25, and 29 gauge patient concerns. Questions 10, 15, 17, 24, and 30 focus on sleep quality, and items 18, 20, 27, 28, and 31 address social difficulties. Lastly, items 1 through 8 pertain to the level of bother or inconvenience experienced by patients [14].
Statistics
Sample size calculation
Due to the limited literature available on patients with pelvic ischemia and LUTS, our sample size estimation was based on only 2 identified studies. The first compared the IPSS between patients with pelvic ischemia and age-/sex-matched controls (total score for patients, 11±3; total score for controls, 8±2; P=0.02) [7]. The second study compared the IPSS before and 6 months after revascularization in patients with iliac artery obstruction, reporting a 4-point reduction. Specifically, pre-surgery IPSS and IPSS-QoL scores were 13.7±8.4 and 3.3±1.0, respectively, while the post-surgery IPSS was 8.1±6.9 (P<0.001) and the post-surgery IPSS-QoL was 2.1±1.1 (P<0.001) [15]. To achieve comparable results, our sample size was estimated to include 30 patients and 30 controls. We allowed for additional recruitment of 10% to account for potential dropouts. A power analysis based on the presence of moderate-to-severe LUTS in patients and controls yielded a power of 87.19%.
Study outcomes
The primary outcome involved comparing IPSS values between patients and controls. Secondary outcomes included:
(1) Comparison of baseline IIEF and OAB-q scores between patients and controls
(2) Comparison of clinical results (uroflow and ultrasound parameters, blood test results) between patients and control participants
(3) Identification of independent factors contributing to LUTS, sexual dysfunction, and DO via multivariate analysis of statistically significant results as dependent variables; this analysis was conducted separately for patient and control groups.
(4) Clinical results and questionnaire scores, compared before and after endovascular revascularization for all patients and patient subgroups including those with unilateral or bilateral obstruction and those with Leriche syndrome
(5) Comparison of clinical results and questionnaire scores between patients with Leriche syndrome and those with either unilateral or bilateral obstruction
(6) Comparison of UDS findings before and after intervention in patients with IPSS>7
Statistical analysis
Analysis was conducted using IBM SPSS Statistics ver. 27.0 (IBM Co., Armonk, NY, USA). Descriptive statistics were reported as mean±standard deviation and percentages. A normality test was employed to determine whether variables were parametric or non-parametric, and the appropriate analyses were conducted accordingly. Parametric results were presented as mean and standard deviation, while nonparametric results were reported as median and interquartile range (IQR). Statistical significance was established at a level of 0.05 with a 95% confidence interval.
Endovascular Revascularization Procedure
All procedures were conducted electively in a fully equipped hybrid operating theater with a portable C-arm (Pulsera, Philips, The Netherlands). Access to 1 or both femoral arteries, the latter in cases of bilateral lesions, was obtained under local anesthesia or monitored anesthesia care using a 6F sheath. Systemic heparinization (75–100 IU/kg) was then induced. Angiography was performed to visualize the steno-occlusive lesions. A stiff hydrophilic guidewire (Radifocus, Terumo Corp., Tokyo, Japan) was employed to traverse the stenosis or obstruction, assisted by a catheter (Bern, Imager II; Boston Scientific, Marlborough, MA, USA). A balloon-covered stent was deployed to recanalize common iliac artery stenosis or obstruction. In cases of lesions at the aorto-iliac bifurcation, a kissing stent technique was utilized. Final intraoperative angiography was conducted to confirm the patency of the common iliac arteries. All patients were discharged with dual antiplatelet therapy for a minimum of 3 months, followed by lifelong single antiplatelet therapy. The treatment approach for patients with complete obstruction or concurrent obstruction of the abdominal aorta (Leriche syndrome) did not differ from those with >80% iliac artery obstruction.
RESULTS
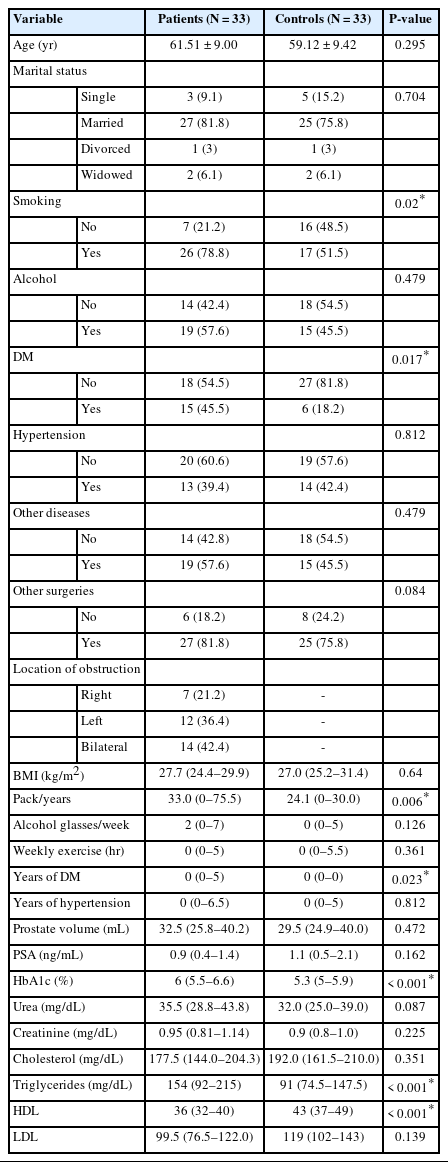
The final sample consisted of 33 male patients and 33 agematched healthy men. Eight patients (22.2%) had IPSS >7 and underwent additional UDS testing. Among the 33 patients, 14 (42.4%) had bilateral iliac artery obstruction, 5 (15.2%) had Leriche syndrome, and the remainder had unilateral common iliac artery obstruction. The demographic information of the participants is presented in Table 1. No significant differences were observed in sociodemographic history between patients and controls, except for pack-years. Similarly, DM was more prevalent in patients compared to controls, and the duration of DM was longer in the patient group. In terms of initial laboratory evaluations, patients had worse hemoglobin A1c and triglyceride levels but better HDL levels (Table 1).
Technical success was achieved for all patients (100%). No adverse events, such as access site hematoma, arterial thrombosis, dissection, or contrast-induced nephropathy, occurred during the peri-operative period. The average hospitalization time was 2.09 days, with a range of 2 to 3 days.
Baseline comparisons between patients and controls demonstrated statistically significant differences in both LUTS and sexual function. Specifically, the IPSS storage, voiding, and total scores, as well as IPSS QoL, were significantly worse in patients compared to controls. Furthermore, patients reported significantly poorer OAB-total, coping, sleep, and bother scores than control participants. The IIEF analysis revealed that erectile function, desire, and satisfaction from intercourse were also significantly lower in patients than in controls. Uroflowmetry and ultrasound results showed a statistically significant difference only in PVR volume between these groups. Voided volume, Qmax, Qave, and voiding time did not exhibit significant differences (Table 2).
Associations Between Iliac Artery Lesions, Confounding Factors, and LUTS/Sexual Function
A multivariate analysis was conducted using all previously mentioned statistically significant variables from the univariate analysis as independent variables while controlling for confounding factors. Iliac artery steno-occlusive disease (P=0.021, β=-0.235) and age (P<0.001, β=-0.467) were independently and negatively associated with erectile function among participants. In contrast, the HDL level was positively associated (P=0.004, β=0.295) with erectile function, and this model accounted for 40.4% of the variance in erectile function (analysis of variance [ANOVA] P<0.001, adjusted R2=0.404). Similarly, iliac artery obstruction (P=0.005, β=-0.320) and age (P=0.002, β=-0.350) were independently and negatively associated with sexual desire, with this model accounting for 23% of the variance (ANOVA P<0.001, adjusted R2=0.23). Only age (P=0.009, β=-0.349) was independently and negatively associated with satisfaction from intercourse, explaining 28.1% of its variance (ANOVA P<0.001, adjusted R2=0.281).
Regarding LUTS, both iliac artery steno-occlusive disease (P=0.009, β=0.324) and alcohol consumption (P=0.049, β=0.238) were independently and positively associated with the IPSS bother score, accounting for 23.6% of its variance (ANOVA P<0.001, adjusted R2=0.236). Additionally, the presence of steno-occlusive disease was independently and positively associated with the IPSS total (P<0.001, β=0.397, ANOVA P<0.001, adjusted R2=0.257), IPSS voiding (P=0.016, β=0.314, ANOVA P=0.014, adjusted R2=0.157), and IPSS storage scores (P=0.002, β=0.375, ANOVA P=0.002, adjusted R2=0.182), explaining 25.7%, 15.7% and 18.2% of their respective variances. Only age (P=0.024, β=0.284) was independently and positively associated with PVR, accounting for 9.8% of its variance (ANOVA P=0.038, adjusted R2=0.098). No significant differences were observed between unilateral or bilateral iliac artery stenoocclusive lesions in any of these analyses. Univariate analysis revealed no significant independent variables other than iliac artery obstruction for patient OAB scores.
Role of Revascularization
The median follow-up time after the intervention was 6 months (IQR, 6–7 months). Comparison of patient scores before and after the intervention showed significant improvements in orgasmic, sexual desire, and erectile function scores, while the median PVR was significantly reduced (Table 3). In patients with moderate-to-severe LUTS (IPSS>7), the IPSS voiding score significantly decreased after the intervention (before, 8± 5.29; after, 4.87±2.79; P=0.026 according to the paired t-test), as well as the orgasmic function score (before: median, 2; IQR, 0–9, after: median, 10; IQR, 6.25–10; P=0.042) according to the Wilcoxon signed-rank test). Regarding urodynamic results, DO and increased bladder sensation significantly improved after the intervention (Table 4).
Subgroup Analysis
Comparison between patients with unilateral and bilateral steno-occlusive disease revealed no significant differences in IPSS, OAB-q, and IIEF scores at either baseline or follow-up (data not shown). Furthermore, subgroup analysis comparing patients with Leriche syndrome to those with either unilateral or bilateral obstruction showed no significant differences in any domain of IPSS, OAB-q, and IIEF. Additionally, comparing pre- and post-surgical revascularization in patients with Leriche syndrome did not reveal significant differences.
DISCUSSION
The primary finding of the present study is that high-grade common iliac artery stenosis (80%) or complete obstruction is an independent risk factor for male sexual dysfunction and LUTS. The presence of such lesions was associated with more severe storage and voiding symptoms, QoL, and urodynamic DO. Erectile function, sexual activity, and satisfaction from intercourse were also negatively affected. By 6 months postoperatively, patients with common iliac artery obstruction experienced significant improvements in erectile function, orgasm, and desire. Similarly, PVR, bladder sensation, and overactivity upon UDS significantly improved. Several factors associated with LUTS and ED were considered as covariates in our analysis [13,16,17]. However, the duration of DM, dyslipidemia, hypertension, and BMI as components of metabolic syndrome, as well as age as a risk factor for LUTS, were not significantly associated with moderate-to-severe symptoms in multivariate analysis. In contrast, the presence of iliac artery steno-occlusive disease was an independent variable regardless of laterality. Concerning patients with Leriche syndrome, we found no significant difference in the burden of LUTS compared to other patients, while the postoperative results did not show improvement of urinary symptoms at the 6-month follow-up.
At present, research on vasculogenic causes of LUTS primarily relies on animal models. In those studies, vascular damage is induced acutely through balloon injury [18]. In contrast, humans typically experience chronic iliac artery obstruction, which tends to manifest in later decades of life and is often accompanied by other comorbidities [19]. Despite the differences in pathophysiological mechanisms of arterial atherosclerotic lesions, the present study suggests that common iliac artery steno-occlusive disease may be a potential risk factor for ED and LUTS in men.
Currently, only 1 human study has been published investigating the association between pelvic ischemia due to abdominal and common iliac artery obstruction and LUTS. In that study, Antunes-Lopes et al. [7] validated preexisting animal models. They found that the IPSS was higher in patients with chronic pelvic ischemia, with a significant mean difference of 3 points relative to healthy individuals. However, their sample size was significantly smaller (n=13) than ours (n=33) and was also limited by age, as it examined only male patients older than 60 years. Moreover, they did not use a specific tool to assess the presence and severity of OAB, and they demonstrated no significant difference between patients and controls in IPSS storage and voiding subscores, along with OAB-q scores, in contrast to our study. In another study, LUTS and ED were investigated before and after revascularization of obstructive lesions distal to iliac vessels. Twenty-six of 55 patients underwent stenting in addition to balloon angioplasty. The average IPSS, IPSS-QoL, and IIEF had improved significantly at 1 and 6 months. The improvement in IPSS was significantly greater in patients with lesions located in the proximal internal pudendal artery or iliac arteries compared to those with lesions located more distally. No differences in IIEF-5 scores were observed [15]. These results align with our study findings. However, their intervention was more peripheral than ours, including a wide variety of treatment targets (the proximal internal pudendal, iliac, distal internal pudendal, and penile arteries). Furthermore, only the IPSS and IIEF-5 were used to evaluate lower urinary tract function, while other causes of LUTS, such as benign prostatic hyperplasia (BPH), were not excluded or controlled for.
Endovascular revascularization may also positively impact sexual function. Epidemiological studies have suggested a link between sexual dysfunction and coronary artery disease. In fact, up to 75% of patients with ED exhibit stenosis of the iliacpudendal-penile arteries [19]. Research on the effects of revascularization on erectile function is limited, but Mazzaccaro et al. [20] reported significant improvements in erectile function among patients who underwent endovascular antegrade revascularization for occlusion, compared to those who received surgical femoral-femoral crossover bypass. Similarly, Gur et al. [21] highlighted the positive impact of endovascular revascularization of common iliac artery occlusions on erectile function. These findings align with the present results, further supporting the therapeutic benefits of endovascular surgery for these individuals.
Regarding the clinical implementation of our results, no indication currently exists for computed tomography angiography, magnetic resonance angiography, or other diagnostic tests for vascular disease in patients with LUTS. However, for vasculogenic ED, the European Association of Urology guidelines recommend further investigation using intracavernous vasoactive drug injection, penile dynamic duplex ultrasonography, penile dynamic infusion cavernosometry and cavernosography, and internal pudendal arteriography. Nevertheless, intracavernous vasoactive drug injection has been reported to be inconclusive as a diagnostic procedure, and dynamic infusion cavernosometry and cavernosography are infrequently used for diagnosing venogenic ED. Furthermore, pudendal arteriography is only performed in patients under consideration for penile revascularization in cases of isolated penile artery stenosis [22]. From our perspective, patients with LUTS or ED and associated risk factors for vascular disease (such as metabolic syndrome) or previous ischemic disease should be asked about the presence of intermittent claudication and staged according to the Fontaine classification. The presence of intermittent claudication or the absence of BPH and other causes of LUTS, such as neurological disease, in these patients may indicate the need for further vascular investigation. Additionally, patients with LUTS and iliac artery obstruction may continue to experience symptoms after BPH treatment. Lastly, physicians treating patients with storage symptoms should consider addressing iliac artery obstruction before pursuing further treatment for BPH.
This study had several limitations. First, the cross-sectional design did not allow for causal conclusions to be drawn. Additionally, although our study sample may seem small, it was adequately powered to address the primary outcome and was larger than previously published studies in the field. However, the number of patients involved in subgroup analyses, particularly those who underwent urodynamic investigation and those with Leriche syndrome, were only 8 and 5, respectively, which prevents the generalization of our results. We also did not examine patients with internal iliac artery obstruction or microvascular obstruction, which are potential causative factors for LUTS and ED. Likewise, we were unable to evaluate compensatory mechanisms of microvascular obstruction present in patients with chronic diseases, such as atherosclerosis. Another major factor in the pathogenesis of male LUTS that cannot be completely excluded in human studies is BPH. To minimize the effect of prostate size on micturition in both patients and controls, we used an IPSS of <8 as a threshold for asymptomatic patients and controls. We also compared prostate volume using transabdominal ultrasound, and although this is not interchangeable with transrectal ultrasound, we found no significant difference in prostate volume between patients and controls. Due to the invasiveness of transrectal ultrasound and the associated patient discomfort, it is not common practice in Greece unless absolute indications are present. Further investigation of LUTS in patients who have undergone transurethral resection of the prostate or other BPH therapies and were subsequently treated with endovascular surgery may be necessary. Lastly, our study included only male patients. Despite this limitation, our findings may encourage further research on the importance of the role of pelvic ischemia in ED and LUTS, as well as the potential therapeutic role of revascularization surgery.
In conclusion, this case-control study is the first to present evidence that common iliac artery steno-occlusive disease is an independent risk factor for LUTS and ED relative to healthy individuals. In our patient cohort, endovascular revascularization surgery significantly improved postoperative erection, desire, and orgasm, suggesting a potential new indication for the treatment of vasculogenic ED. Moreover, patients with moderate or severe LUTS particularly benefited from surgery in terms of voiding symptoms. To our knowledge, this is the first study to perform urodynamic evaluation in humans before and after endovascular revascularization surgery. It is also the first to examine lower urinary tract function in patients with Leriche syndrome before and after surgical revascularization. Future studies with larger sample sizes and longer follow-up durations, utilizing urodynamic investigations before and after intervention, are necessary to draw more robust conclusions. Given the high prevalence of LUTS and ED in patients with atherosclerosis, endovascular revascularization may become a common treatment for vasculogenic LUTS and ED.
Notes
Research Ethics
The study was approved by the local university ethics committee and the Institutional Review Board. It was registered with the Australian and New Zealand Clinical Trial Registry (ACTRN12620000015943). The study was conducted in accordance with the Declaration of Helsinki, and all participants were enrolled after providing written informed consent.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTION STATEMENT
• Conceptualization: EP, IL, AA
• Data curation: EP, SG, KT, IA, PK, IL
• Formal analysis: EP, PK
• Methodology: EP, AA
• Project administration: IL, AA
• Writing - original draft: EP
• Writing - review & editing: SG, KT, IA, PK, IL, AA