Outcomes and Complication Rates of Cuff Downsizing in the Treatment of Worsening or Persistent Incontinence After Artificial Urinary Sphincter Implantation
Article information
Abstract
Purpose
This study investigated the functional outcomes and complication rates of cuff downsizing for the treatment of recurrent or persistent stress urinary incontinence (SUI) in men after the implantation of an artificial urinary sphincter (AUS).
Methods
Data from our institutional AUS database spanning the period from 2009 to 2020 were retrospectively analyzed. The number of pads per day was determined, a standardized quality of life (QoL) questionnaire and the International Consultation on Incontinence Questionnaire (ICIQ) were administered, and postoperative complications according to the Clavien-Dindo classification were analyzed.
Results
Out of 477 patients who received AUS implantation during the study period, 25 (5.2%) underwent cuff downsizing (median age, 77 years; interquartile range [IQR], 74–81 years; median follow-up, 4.4 years; IQR, 3–6.9 years). Before downsizing, SUI was very severe (ICIQ score 19–21) or severe (ICQ score 13–18) in 80% of patients, moderate (ICIQ score 6–12) in 12%, and slight (ICIQ score 1–5) in 8%. After downsizing, 52% showed an improvement of >5 out of 21 points. However, 28% still had very severe or severe SUI, 48% had moderate SUI, and 20% had slight SUI. One patient no longer had SUI. In 52% of patients, the use of pads per day was reduced by ≥50%. QoL improved by >2 out of 6 points in 56% of patients. Complications (infections/urethral erosions) requiring device explantation occurred in 36% of patients, with a median time to event of 14.5 months.
Conclusions
Although cuff downsizing carries a risk of AUS explantation, it can be a valuable treatment option for selected patients with persistent or recurrent SUI after AUS implantation. Over half of patients experienced improvements in symptoms, satisfaction, ICIQ scores, and pad use. It is important to inform patients about the potential risks and benefits of AUS to manage their expectations and assess individual risks.
INTRODUCTION
Stress urinary incontinence (SUI) in men primarily occurs as a complication following treatment for prostate disease, significantly impacting their quality of life (QoL). Implantation of an artificial urinary sphincter (AUS) remains the “gold standard” for treating SUI [1]. Despite favorable long-term outcomes and high satisfaction rates, SUI tends to recur after AUS implantation [2,3]. The most common cause of new-onset SUI due to nonmechanical failure is urethral atrophy, which affects 7.9% of patients [2,4]. The underlying pathophysiological mechanism is believed to be urethral tissue hypoxia caused by continuous circular compression from the cuff on the urethra [5,6]. Cuff downsizing, along with other techniques such as applying a tandem or transcorporeal cuff, replacing the cuff, or replacing a pressure-regulating balloon, is a standard therapeutic approach for treating newonset SUI [7-9]. Cuff downsizing has been shown to decrease daily pad usage, reduce the number of severe leakage episodes, and enhance patient satisfaction [7].
There is limited literature—with only 2 available studies— evaluating the outcomes of cuff downsizing in terms of continence outcomes and complication rates during medium to long-term follow-up [7,10]. The aim of the present study was to analyze these parameters precisely and, thus, to determine for which patients cuff downsizing constitutes a relevant therapy option.
MATERIALS AND METHODS
Patient Population
Since 2009, all perioperative and follow-up data for patients who underwent AUS implantation (AMS 800, Boston Scientific Corp., Marlborough, MA, USA) at the University Medical Center Hamburg-Eppendorf have been collected in an AUS database. Prior to the initial implantation, patient evaluations at our center include completing the International Consultation on Incontinence Questionnaire (ICIQ) and a QoL questionnaire, maintaining a voiding diary, undergoing urodynamics, performing a pad weight test, and having a cystoscopy. Patients with detrusor overactivity, bladder capacity less than 300 mL, or detrusor overactivity evident during the first 300 mL of bladder filling on preoperative urodynamic cystometry receive medical treatment and counseling before repeating the examinations and proceeding with surgery. For new-onset incontinence occurring after AUS implantation, assessments include urinalysis, cystoscopy to evaluate erosion, ICIQ and QoL questionnaires, pad weight test, anteroposterior radiography, and, if indicated, a repeated urodynamic test. If a nonmechanical failure (typically urethral atrophy) is identified as the cause, the patient is offered sphincter downsizing. Our center does not perform changes to a larger balloon.
Surgical Procedure
Surgery was performed by 2 experienced (high-volume) surgeons following a standardized protocol. All patients were administered perioperative intravenous antibiotic therapy, consisting of cephalosporin and gentamicin, for 5 days.
Patients were positioned in the lithotomy position. A perineal approach was employed to expose the in situ AUS device. Preparation was carried out using monopolar electrocautery to prevent mechanical damage to the AUS. The closed cuff was opened and removed. The urethral circumference was then measured using the AUS accessory kit. The cuff was positioned in the same location as the previous one and connected to the pump using Quick Connectors (Boston Scientific Corp.). If the AUS device was older than 5 years, the entire system was replaced in accordance with our institutional standards.
The AUS system was deactivated, and a 12F Foley catheter was inserted and left in place for 3 days. Following the catheter’s removal, the AUS device was activated on day 3 under radiological supervision. The functional outcome was assessed through uroflowmetry, postvoid urine measurement, pad stress test, and clinical examination.
Follow-up
Follow-up was conducted in accordance with our institutional protocol. Patients remained hospitalized until day 3. Patients were advised to return to our hospital at 6 and 24 months, and every 2 years thereafter for clinical examination, pad weight test, and radiography. In the interim, patients were monitored by their private practice urologist, who maintained close communication with us and, if necessary, arranged for spontaneous visits to our consultation or a 24-hour emergency room.
Study Endpoint
The primary endpoint of the study was the continence rate following cuff downsizing, assessed by the number of pads used per day and the ICIQ score. The ICIQ score ranges were as follows: 1–5 (slight), 6–12 (moderate), 13–18 (severe), and 19–21 (very severe) [11]. The secondary endpoints included complication rates, evaluated using the Clavien-Dindo classification, with a focus on infections and urethral erosion leading to subsequent explantation of the device. Additionally, the satisfaction rate was measured by assessing the QoL on a 6-point scale, according to the QoL questionnaire.
Statistical Analysis
Descriptive statistics, including measures of central tendency and variability, as well as linear regression, were carried out using R version i386 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Patient Characteristics
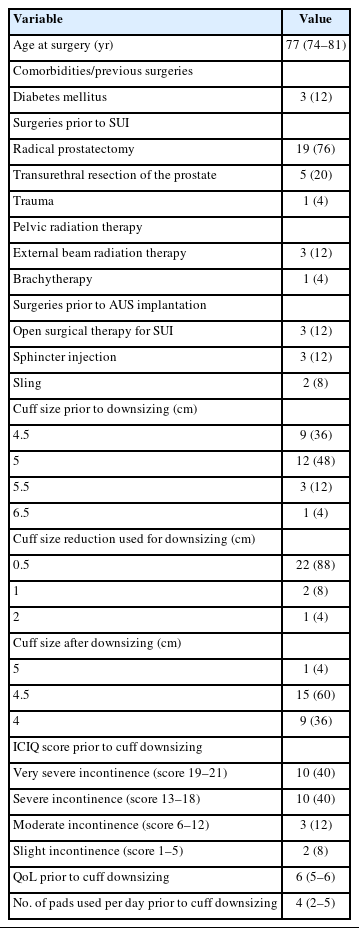
Out of the 477 patients included in our study, we identified 25 male patients (5.2%) with a median age of 77 years (interquartile range [IQR], 74–81 years) who underwent cuff downsizing due to new-onset SUI following AUS implantation. The median follow-up period was 4.4 years (IQR, 3–6.9 years).
A summary of patient characteristics is presented in Table 1. In total, 19 patients (76%) had undergone radical prostatectomy before initial AUS implantation, while 5 patients (20%) had undergone transurethral resection of the prostate, and 1 patient initially experienced urethral trauma. Four patients (16%) had a history of pelvic radiotherapy. In terms of secondary diagnoses, 3 patients (12%) had diabetes. Prior to AUS placement, 8 patients (32%) had undergone an SUI procedure, which included open surgical therapy of SUI, sling, or sphincter injection.
The median cuff size prior to downsizing was 5 cm (IQR, 4.5–5 cm), while the median cuff size following downsizing was 4.5 cm (IQR, 4–4.5 cm). In 21 patients (84%), the cuff size was reduced by 0.5 cm, in 2 patients (8%) by 1 cm, and in 1 patient (4%) by 2 cm. The most common cuff sizes before downsizing were 5 cm (44%) and 4.5 cm (36%).
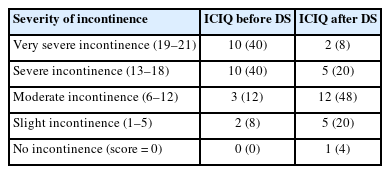
Prior to downsizing, 20 (80%) of the patients experienced very severe or severe SUI, with 40% having an ICIQ score of 19–21 and 40% having an ICIQ score of 13–18. Additionally, 3 of the patients (12%) had moderate SUI (ICIQ score 6–12), and 2 of them (8%) had mild SUI (ICIQ score 1–5).
The median score on the QoL questionnaire was 6 (IQR, 5–6), and the median number of pads used per day (IQR) was 4 (IQR, 2–5).
Complication Rates
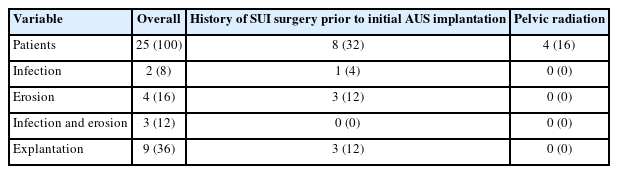
Infection occurred in 2 patients (8%) and erosion in 4 patients (16%), resulting in the explantation of the device after cuff downsizing in the subsequent course. Four patients (16%) experienced an infection along with urethral erosion. The median (IQR) time to event was 14.5 months (5.8–26 months). Among the patients with complications, 4 (16%) had undergone SUI surgery prior to downsizing. None of these patients had a history of radiotherapy. A summary of the complication rates can be found in Table 2.
Functional Outcomes
With respect to ICIQ scores, after cuff downsizing, 2 patients suffered from incontinence.
Fourteen patients (56%) reported a recurrence of SUI after a median of 3.5 months (IQR, 0–19.5 months). In the majority of these patients, SUI occurred within the first 6 months following downsizing, while in 4 of them (16%), it occurred after 24 to 38 months.
In terms of daily pad usage, 13 patients (52%) experienced a reduction of 50% or more. There was no significant pad reduction (20% or less) in 6 patients (24%). After the intervention, 3 patients (12%) required more pads per day. The median daily pad usage before downsizing was 4 (IQR, 2–5), and after downsizing, it was 2 (IQR, 1–2.5).
In the QoL questionnaire, 76% of patients (n=19) reported a QoL score of ≥5 out of 6 points prior to downsizing. Following downsizing, 56% of patients (n=14) experienced an improvement of ≥2 points. Only 1 patient (4%) demonstrated a decline, with a QoL score of 3 before and 4 after downsizing. The functional outcomes are summarized in Table 3.
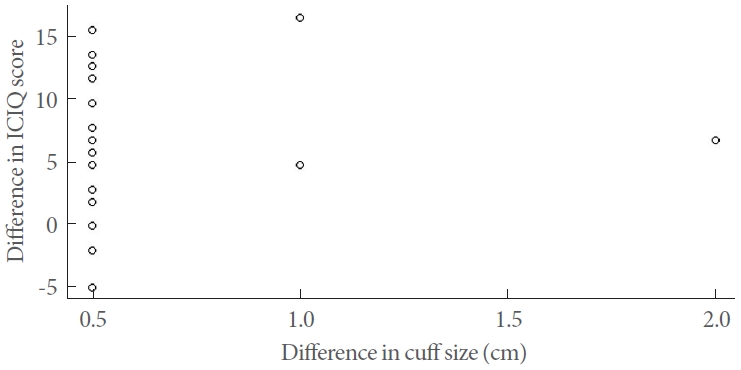
Linear regression analysis was used to assess the relationship between the difference in cuff size and the change in ICIQ score. However, our findings revealed no significant correlation between these 2 variables (P=0.48, R2=0.022, slope=2.64). The individual relationship between the difference in cuff size and the change in ICIQ score is depicted as a scatter plot in Fig. 1.
DISCUSSION
New-onset incontinence due to nonmechanical failure following AUS implantation is predominantly caused by urethral atrophy [12]. Various treatment options have been described in the literature, including cuff downsizing, cuff replacement, transcorporeal cuff placement, tandem cuff placement, and pressureregulating balloon replacement with higher pressure [7,13-17]. However, due to insufficient data, there is no consensus on the optimal treatment approach. Pressure-regulating balloon replacement is associated with a high rate of device revision within 2 years and poor functional outcomes, which is why this technique is not performed at our center. In this study, we excluded patients who underwent tandem cuff or cuff replacement and focused on cuff downsizing. With only 3 studies available, there is a lack of data to evaluate outcomes following cuff downsizing in terms of SUI and complication rates [7,10]. Additionally, there is a scarcity of data on medium- to long-term outcomes. In 2003, Saffarian et al. [7] published their findings on cuff downsizing as a treatment for recurrent incontinence due to urethral atrophy in 17 patients. They reported an average time of 31 months to develop urethral atrophy after the initial device implantation. The follow-up period was 22 months. All patients received a 4.5-cm cuff. The procedure led to a decrease in pad usage (from 3.9 to 0.5 per day), a reduction in severe leakage episodes, and increased patient satisfaction.
Linder et al. [3] reported a median time of 4.7 years to urethral atrophy in a long-term follow-up of 1,082 cases after primary AUS placement. However, it is important to consider that advancements in AUS surgery have occurred since the publication of these articles. With the introduction of the 3.5-cm cuff, it is worth discussing whether these studies may have potential confounding factors due to cuff oversizing prior to the 3.5-cm cuff era. It is also important to note that our study specifically excluded patients who underwent AUS implantation before 2009, and our institution did not use 3.5-cm cuffs due to concerns about urethral erosion, as demonstrated in the subsequent study by Queissert et al. [18].
Krughoff et al. [19] recently published a retrospective study examining the effects of cuff downsizing in cases of subcuff atrophy among patients with post-prostatectomy incontinence. Unlike the present study, their research included a total of 90 patients who underwent downsizing for various reasons, such as mechanical failure. Of these, 34 patients had cuff downsizing due to urethral atrophy. The median follow-up period was 3.4 years. Incontinence outcomes were evaluated using pad usage, subjective measures, and American Urological Association Symptom Score ratings.
To the best of our knowledge, no other study has specifically examined the median time to recurrence of incontinence following cuff downsizing during a medium- to long-term followup period. Our study focused specifically on patients who underwent cuff downsizing due to recurrent or persistent SUI following AUS implantation. The assessment of outcomes was conducted using standardized questionnaires (ICIQ and QoL) and pad usage.
In 2017, Linder et al. [10] published the results of 69 revision procedures for urethral atrophy following AUS implantation, comparing tandem cuff placement (56 patients, 82%) with single cuff downsizing (12 patients, 18%). The median follow-up period was 2.21 years. Complications necessitating tertiary intervention occurred in 19 patients (27.5%) during the subsequent course. These complications included device infection/urethral erosion in 12 patients (17.4%), urethral atrophy in 4 patients (5.7%), and device malfunction in 3 patients.
Urethral atrophy is the primary cause of emergent SUI, resulting in cuff downsizing and affecting 7.9% of patients [2,4]. The pathophysiological mechanism is thought to involve pressure necrosis and hypoxia of the urethral tissue. It can be hypothesized that a smaller cuff may lead to a greater degree of tissue atrophy or erosion over time.
Our results show that cuff downsizing led to improved continence, as evidenced by a lower use of pads per day (a reduction of ≥50% in 52% of patients), a decreased number of cases with very severe or severe SUI, and enhanced patient satisfaction. In fact, 56% of patients experienced an improvement in QoL by ≥2 points, which is consistent with the findings of Saffarian et al. [7]. We observed a transition from very severe to moderate and slight SUI, with a reduction of 32% for very severe and 20% for severe SUI. However, very severe and severe SUI (28%) remained relatively common after downsizing, and 24% of patients did not experience a significant pad reduction (≤20%). Notably, 3 patients required more pads per day, and only 1 patient achieved complete continence. Similarly, Saffarian et al. [7] reported a decrease in pad usage, although the continued use of at least 1 safety pad was still necessary. In our study, 16% of patients experienced a recurrence of SUI between 24 and 38 months following cuff downsizing. This may be attributed to the progression of urethral atrophy; however, the majority of patients reported SUI recurrence within the first 6 months postsurgery.
An important point to keep in mind is the high risk of complications in subsequent stages, particularly given that the explantation rate due to infection or erosion is 36%. This typically occurs after a median of 14.5 months. Linder et al. [10] also reported comparable findings, with high complication rates of approximately 27.5%.
According to a retrospective study by Lai et al. [4] involving 270 patients, the complication rates after primary AUS implantation were as follows: 5.5% for infection (median time, 3.7 months), 6% for erosion (median time, 19.8 months), 9.6% for urethral atrophy (median time, 29.6 months), 6% for mechanical failure (median time, 68.1 months), and 27.1% for surgical removal or revision (median time, 14.4 months). In comparison to the explantation rates due to infection, erosion, or urethral atrophy (21.1%) after primary AUS implantation, our results demonstrate a 1.7-fold higher risk following secondary implantation, such as cuff downsizing, with the same time to event [4].
As the risk of device explantation has been shown to be particularly high in patients with diabetes mellitus, a history of pelvic radiotherapy, or previous urethral or SUI surgery, we further analyzed these factors in our cohort [13,20]. Only 4 of the included patients (16%) had a history of radiotherapy, and none of them experienced relevant complications after cuff downsizing. This is consistent with our recently published study showing no significant difference in complication rates between patients with or without a history of pelvic radiotherapy [21]. However, it is important to note that 3 of the 8 patients (37.5%) included who had a history of other incontinence surgery before initial AUS implantation experienced a complication that led to explantation of the device after cuff downsizing. Additionally, of the 9 patients (36%) who had complications, onethird had a history of SUI. The correlation between a surgical history of SUI and revision surgery is consistent with the current literature. Lai et al. [20] have demonstrated a 4-fold higher risk of future cuff erosion and explantation for reimplantation cases compared to primary cases. Wang et al. [22] reported that more than 50% of patients required further revisions at a median time of 2 years after reimplantation, supporting these findings.
Considering the high complication and persistence rates of severe SUI, the potential for significant improvement in personal satisfaction and QoL should be weighed against these risks. Indeed, we observed an enhancement in patient satisfaction, with 56% of patients experiencing an improvement in QoL by ≥2 out of 6 points. However, nearly half of the patients experienced minimal or no improvement, which did not correlate with the severity of SUI or the number of pads used per day. We suspect that patients’ subjective expectations of continence outcomes may influence postoperative outcomes and satisfaction.
One limitation of this study is its retrospective nature, which may introduce bias towards more compliant and/or motivated patients who choose to undergo AUS downsizing. Additionally, the small number of patients is a drawback, as the research question is highly specific to a rare intervention. However, to our knowledge, this is 1 of only 3 existing studies that analyze outcomes after cuff downsizing, and it is the only study that examined medium- to long-term follow-up and evaluated complication rates, continence outcomes, and patient satisfaction using standardized outcome measurements (ICIQ and QoL).
In summary, our study demonstrated that cuff downsizing can enhance SUI and patient satisfaction in over half of cases, with more than 50% of patients experiencing a substantial decrease in pad usage or an improvement in their ICIQ scores. Before downsizing, 76% of patients reported a QoL score ranging from 5 to 6 points; however, after downsizing, 56% of patients exhibited an improvement of more than 2 points.
While complete continence may not be achievable in all cases, reducing the cuff size can still offer significant benefits to many patients. However, it is crucial to recognize that the procedure carries a high risk of complications, with a 36% chance of AUS explantation within 5 years. Furthermore, a considerable number of patients may continue to experience moderate to severe SUI after the procedure. As a result, it is important to evaluate the risks involved in each individual case when considering cuff reduction. Patients must be provided with appropriate counseling. Cuff reduction should be particularly contemplated for patients with severe SUI and a high disease burden.
Notes
Research Ethics
According to local law, retrospective studies do not require Institutional Review Board approval.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTION STATEMENT
• Conceptualization: SW, TAL, VM
• Data curation: SW, TAL, VM
• Formal analysis: SW
• Methodology: SW
• Project administration: SW
• Visualization: SW
• Writing - original draft: SW
• Writing - review & editing: SW, TAL, OB, PG, MWV, MF, RD, VM