The Ketone Bridge Between the Heart and the Bladder: How Fast Should We Go?
Article information
Abstract
Metabolic syndrome (MS) is associated with both cardiovascular and bladder dysfunction. Insulin resistance (IR) and central obesity, in particular, are the main risk factors. In these patients, vicious pathological cycles exacerbate abnormal carbohydrate metabolism and sustain an inflammatory state, with serious implications for both the heart and bladder. Ketone bodies serve as an alternative energy source in this context. They are considered a “super-fuel” because they generate adenosine triphosphate with less oxygen consumption per molecule, thus enhancing metabolic efficiency. Ketone bodies have a positive impact on all components of MS. They aid in weight loss and glycemic control, lower blood pressure, improve lipid profiles, and enhance endothelial function. Additionally, they possess direct anti-inflammatory, antioxidant, and vasodilatory properties. A shared key player in dysfunction of both the heart and bladder dysfunction is the formation of the NLRP3 inflammasome, which ketone bodies inhibit. Interventions that elevate ketone body levels—such as fasting, a ketogenic diet, ketone supplements, and sodium-glucose cotransporter 2 inhibitors—have been shown to directly affect cardiovascular outcomes and improve lower urinary tract symptoms derived from MS. This review explores the pathophysiological basis of the benefits of ketone bodies in cardiac and bladder dysfunction.
INTRODUCTION
Energy metabolism is fueled by various substrates, including carbohydrates, fatty acids, amino acids, and ketone bodies. While all of these substrates ultimately generate the energy molecule adenosine triphosphate (ATP), their metabolic pathways are distinct and have significant differences [1]. An imbalance in these metabolic pathways can have detrimental effects on human health. Metabolic syndrome (MS) is one such example [2]. MS affects one-quarter of the global population, constituting a global pandemic [3]. A diagnosis of MS must include at least 3 of the following cardiovascular risk factors: elevated fasting glucose levels, hypertension, high triglyceride levels, reduced high-density lipoprotein (HDL) cholesterol levels, and central obesity [4]. These components of MS are known to interact within a shared pathophysiology, predisposing individuals to not only an increased cardiovascular risk but also to noncardiovascular diseases [5-7]. In this context, urologic symptoms have been associated with MS [8]. Therefore, understanding the relationship between the components of MS, cardiovascular health, and urologic health is of the utmost importance.
In this paper, we explore the impact of MS on the cardiovascular and urinary systems, as well as the potential benefits of ketone bodies as an alternative energy source in mitigating these adverse effects. We also review the effects of currently available interventions that modulate serum ketone body concentrations.
CARDIOVASCULAR IMPACT OF METABOLIC SYNDROME
Insulin resistance (IR) and obesity, particularly visceral adiposity, are common in patients with MS and are the primary predictors of cardiovascular mortality associated with this syndrome [2, 4, 6]. IR is linked to the disruption of the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) pathway, which affects the expression of glucose transporter type 4, thereby reducing glucose metabolism in muscle and fat cells [7]. Consequently, fat cells resort to lipolysis, releasing free fatty acids (FFAs) that are re-esterified in the liver. This process results in elevated triglyceride levels and subsequent increases in very low-density lipoprotein (LDL) and HDL, which accounts for the dyslipidemia profile observed in MS patients [2]. Furthermore, the dysfunction of the PI3K-Akt pathway leads to reduced phosphorylation of nitric oxide synthase (eNOS), diminishing nitric oxide production, which in turn causes endothelial dysfunction and hypertension [7]. The increase in visceral adiposity also triggers an overproduction of proinflammatory adipokines and a decrease in anti-inflammatory adipokines, as well as activating the reninangiotensin system [9]. These effects contribute to the persistence of IR and endothelial dysfunction, creating a self-perpetuating cycle that exacerbates each condition and increases the risk of cardiovascular disease [2].
THE IMPACT OF METABOLIC SYNDROME ON THE BLADDER
The impact of MS on the urinary system is increasingly recognized [10]. Several large population-based studies have linked lower urinary tract symptoms (LUTS) with each of the MS components. The Flint Study found an association between LUTS and both hypertension and diabetes in African American males [11]. Similarly, the HUNT study identified diabetes, body mass index, and waist-to-hip ratio as risk factors for LUTS [12]. Although not formally a component of MS, physical inactivity is a known risk factor for all MS components and has been consistently associated with LUTS in several studies [13, 14]. Moreover, the severity of LUTS correlates with several MS components [5]. Indeed, all MS components are linked to pathological changes in the bladder. Obesity and hyperlipidemia are associated with systemic chronic inflammation, evidenced by bladder leukocyte infiltration and locally enhanced cytokine expression [15]. High glucose concentrations and hyperinsulinemia contribute to oxidative stress, insulin-like growth factor-1–driven mitogenic effects, and increased bladder sympathetic tone [10, 16]. Additionally, hypertension is closely related to the incidence of overactive bladder (OAB) due to elevated sympathetic tone and has been shown to increase hypoxia in the urothelium and detrusor muscle [17]. Kim et al. [18] demonstrated that the incidence of urological diseases in metabolically unhealthy obese individuals within the Korean population was significantly higher than in metabolically unhealthy nonobese individuals or in metabolically healthy obese individuals. These findings highlight the significant role of metabolic disease in influencing bladder function.
Evidence from animal models has detailed the underlying alterations that link MS with LUTS. Myocardial infarction-prone Watanabe heritable hyperlipidemic rabbits exhibit OAB with nonvoiding contractions, which may be due to a reduced area of detrusor smooth muscle cells and urothelial thickness, as well as an increase in peptidergic neurons [19]. Obese B6.VLepob/J mice, used as a model for MS, display increased urinary bladder frequency and voiding impairment, along with an increased volume and inflammation of the prostate [20]. Additionally, in a rabbit model of MS induced by a high-fat diet, animals exhibited bladder fibrosis with signs of hypoxia and inflammation [21]. Although the mechanisms are not fully understood, the association between MS and LUTS appears to be partially related to overactivity of the RhoA/Rho-kinase pathway [21, 22]. In MS-related LUTS, overactivity of the estrogen receptor increases RhoA/ROCK signaling, which promotes bladder overactivity [22]. Within this pathway, androgens may contribute to phosphodiesterase type 5 modulation, enhancing RhoA membrane translocation and ROCK overexpression in bladder smooth muscle cells, thereby promoting detrusor contractility and contributing to bladder inflammation and fibrosis [23]. Beyond the estrogen and androgen-dependent mechanisms, MS-related LUTS may also be associated with impaired neuronal activity. Obese Zucker rats exhibit impaired excitatory neurotransmission due to a decrease in cannabinoid receptor expression in the nerve fibers of the urinary bladder [24].
KETOGENESIS
Under conditions of low carbohydrate availability, such as fasting or a low carbohydrate diet, or when insulin action is impaired (as in IR), fat stores are broken down through lipolysis [25]. The FFAs released during this process are utilized by the liver to produce ketone bodies—acetone, acetoacetate, and β-hydroxybutyrate (β-OHB)—via the metabolic pathway of ketogenesis. This pathway involves the use of acetyl-CoA, which is derived from the β-oxidation of FFAs [26]. Ketone bodies are then released into the bloodstream and can be used as energy sources by tissues outside the liver, where they re-enter the Krebs cycle [27]. The production of ketone bodies is suppressed by insulin and stimulated by glucagon and adrenaline, which regulate the activity of key enzymes involved in ketogenesis [27]. Recently, there has been a surge of interest in ketogenesis due to the beneficial effects of ketone bodies as an “alternative fuel” for tissues outside the liver. β-OHB, being the predominant ketone body in circulation, is the focus of most research in this area [25].
Ketogenesis and Cardiovascular Health
The heart derives most of its energy from fatty acid β-oxidation, which accounts for 50%–65%, and from the oxidation of pyruvate that originates from glucose, which contributes 30%–50% [28]. However, the heart exhibits remarkable metabolic flexibility; during states of ketosis, such as fasting, it can utilize ketone bodies for energy. Likewise, cardiomyocytes in patients with type 2 diabetes also metabolize ketone bodies as an energy source [29].
Ketone bodies have been found to be a more efficient energy source than carbohydrates or fatty acids, as they require less oxygen consumption for the same level of ATP production [28]. Consequently, they have been regarded as a “super-fuel” for the heart [29]. Beyond energy efficiency, ketone bodies also play direct beneficial roles at the subcellular level. β-OHB increases eNOS activity and enhances myocardial blood flow through direct vasodilation [30, 31]. In animal models, β-OHB has been shown to decrease infarct size and reduce ischemia/reperfusion injury. It also significantly reduces myocardial apoptosis and oxidative stress [32]. Furthermore, β-OHB exhibits potent antiinflammatory effects by inhibiting the formation of the NODlike receptor protein 3 inflammasome (NLRP3 inflammasome), a key mediator in the pathological progression of cardiovascular diseases [33, 34]. Notably, β-OHB has been found to reduce maladaptive cardiac remodeling in animal models of heart failure [30]. In humans, supplementation with β-OHB has been shown to lower levels of glucose, FFAs, and triglycerides [35] (Fig. 1). Given these beneficial effects, interventions that regulate the formation of ketone bodies are gaining increasing interest in the context of cardiovascular health and MS.
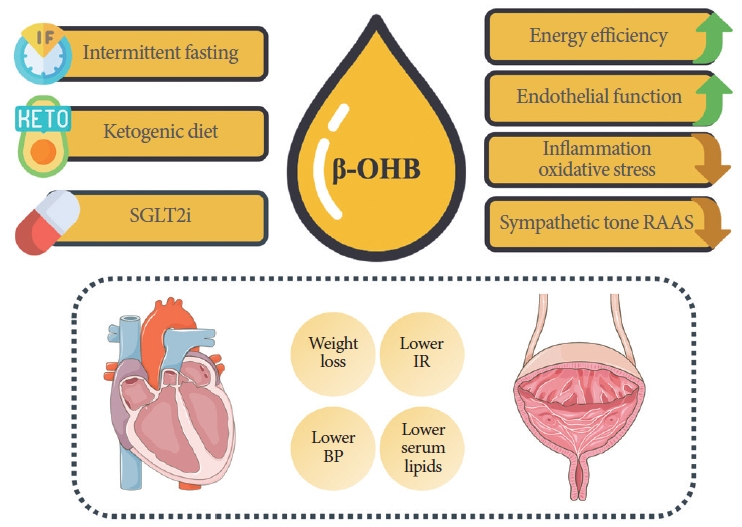
Effects of interventions that increase ketone bodies levels. β-OHB, β-hydroxybutyrate; SGLT2i, sodium-glucose cotransporter 2 inhibitors; RAAS, renin-angiotensin-aldosterone system; BP, blood pressure; IR, insulin resistance. This figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license (https://creativecommons.org/licenses/by/3.0/) and using images from Flaticon.com.
Ketogenesis and Bladder Health
Ketogenesis has not been extensively studied in the context of urological health. However, managing MS components may be beneficial for LUTS derived from MS. For example, visceral adiposity is associated with an OAB [36], and the metabolic effects of ketone bodies might be helpful. Ketone bodies also have a direct impact on the lower urinary system. β-OHB acts on free fatty acid receptor 3 (FFAR3, also known as GPR41), which is expressed in the urothelium and, to a lesser extent, in the detrusor muscle. In urothelial carcinoma, both β-OHB [37] and FFAR3 are found to be highly expressed [38]. Their elevated levels may be associated with protective mechanisms, as β-OHB regulates gene expression by inhibiting histone deacetylases [39], and FFAR3 activation has anticarcinogenic effects [40]. As previously mentioned, β-OHB has a potent effect on the NLRP3 inflammasome [39]. In the bladder, the NLRP3 inflammasome is associated with various pathologies that induce LUTS. It triggers bladder dysfunction in the aging bladder and in the diabetic bladder [41, 42]. In the latter, it is also associated with bladder denervation, which is characteristic of patients with diabetes [42]. Additionally, in models of bladder outlet obstruction, the NLRP3 inflammasome has been shown to be a primary mediator of bladder decompensation, leading to end-stage damage and fibrosis of the detrusor muscle [43]. This has led some researchers to hypothesize that the NLRP3 inflammasome may be a key factor in the transition from an OAB to an underactive bladder (UAB) phenotype, as seen in the aforementioned models [42, 43]. Furthermore, the NLRP3 inflammasome is also implicated in bladder impairment and the development of pain in animal models of interstitial cystitis/bladder pain syndrome (IC/BPS) [44]. Its inhibition by β-OHB resulted in pain improvement in an animal model of spinal cord injury [45]. Therefore, the influence of β-OHB on the NLRP3 inflammasome makes it a promising therapeutic agent for bladder dysfunction (Fig. 1). However, its effects have yet to be validated.
INTERMITTENT FASTING
Intermittent fasting (IF) involves voluntary periods of abstaining from food and drink. This term encompasses a variety of diets [46, 47], all of which ultimately increase ketone body levels during the fasting period.
IF and Cardiovascular Health
IF benefits cardiometabolic health by addressing the various components of MS. IF promotes significant weight loss and a reduction in waist circumference, which corresponds to a decrease in total fat mass [48, 49]. While some of these effects may be due to a lower caloric intake associated with IF [49], the cardiometabolic benefits of IF persist even without a reduction in calorie consumption [48]. Additionally, IF positively influences glucose and insulin levels [49]. Restricting eating to certain hours of the day may enhance the alignment of metabolism with the body’s circadian rhythms [46]. Moreover, during fasting periods, the use of ketone bodies generates fewer reactive oxygen species, thereby reducing oxidative stress in both the pancreas and target organs. This leads to improved insulin secretion and increased peripheral insulin sensitivity [48, 49]. The impact of IF on the lipid profile is more contentious. Some studies have observed reductions in the LDL/HDL ratio and serum triglycerides [48]. However, a recent meta-analysis found no significant effect of IF on the lipid profile [49]. The lack of consistent findings may be due to the substantial heterogeneity among studies examining different types of IF diets. Lastly, IF lowers blood pressure through complex and multifactorial mechanisms. These include a decrease in sympathetic nervous system activity and an increase in parasympathetic tone, as well as a reduction in the activity of the renin-angiotensin-aldosterone system (RAAS) [47, 50].
Another cardiovascular benefit of IF is related with the regulation and favorable changes in the diversity of the gut microbiome [47]. Obese individuals tend to show a less diverse gut microbiome and strains that allow more energy extraction from the diet [46, 47]. Dysbiosis is also linked to cardiovascular risk factors, such as atherosclerosis, hypertension, heart failure, chronic kidney disease, obesity, and type 2 diabetes mellitus [51]. IF led to an enrichment of Parabacteroides distasonis and Bacteroides thetaiotaomicron, which correlated with cardiovascular risk factor modification in humans [52].
IF and Bladder Health
Many cardiovascular benefits of IF can be applied to the treatment of LUTS. Controlling the RAAS axis may be advantageous, as this system has been found to be overactive in patients with benign prostatic hyperplasia (BPH) [53]. Additionally, the glycemic control achieved through IF may positively influence the progression of BPH/LUTS [54]. However, the most significant impact of IF may be on inflammation, a common feature of LUTS associated with OAB, UAB, and MS [15]. IF reduces levels of the proinflammatory molecule soluble intercellular adhesion molecule-1, thereby decreasing the risk of atherosclerosis by regulating leukocyte-endothelial attachment [55]. Moreover, IF lowers the number of circulating inflammatory cells and modulates the NLRP3 inflammasome, without compromising the immune response in healthy individuals [56, 57]. Consequently, IF could play a role in the symptomatic improvement of patients with OAB and UAB. A case report of a patient with ulcerative colitis, a condition often associated with OAB, demonstrated functional enhancement of inflammation-mediated symptoms following an IF regimen [58], indicating that visceral inflammation can be ameliorated by IF. Furthermore, OAB shares additional characteristics with MS, such as IR and reduced levels of HDL cholesterol, which may also improve with an IF diet [59].
IF also modulates the immune response. IF elevates the levels of ketone bodies, specifically β-OHB and acetoacetate, which in turn trigger a CD8+ T-cell immune response against infections and cancer cells by modulating histone acetylation [60]. Cytotoxic CD8+ T cells are key players in the anti-tumor immune response to bladder cancer [61]. The significance of these cells is underscored by the recent identification of CD8+ T-cell infiltration-related molecular signature clusters, which serve as a prognostic tool with strong clinical potential for identifying patients at high risk of bladder cancer [62]. Therefore, IF may have a significant clinical impact on the treatment of bladder cancer. In fact, recent evidence has demonstrated that IF can inhibit epithelial ovarian tumors in a mouse model by enhancing the activity of both CD8+ T and CD4+ T helper cells [63].
Additionally, IF may confer benefits for urinary pathologies by modulating the interplay among diet, the immune system, and the gut microbiota. Pain syndromes such as chronic prostatitis/chronic pelvic pain syndrome and IC/BPS have been linked to fecal dysbiosis [64]. Since IF can aid in the restoration of the gut microbiome, it may also alleviate the pain experienced by these patients. Furthermore, in a mouse model of urinary incontinence caused by sciatic nerve lesions, an IF diet influenced the metabolism of gram-positive bacteria. This led to the release of molecules that promote neutrophil chemotaxis, resulting in the regeneration of the axonal sciatic nerve [65]. Such an effect could potentially influence bladder activity.
Although IF acts on several pathophysiological aspects of bladder dysfunction, to our knowledge, no studies have directly addressed its effects on LUTS. Further investigation may reveal promising results.
KETOGENIC DIET AND KETONE SUPPLEMENTS
Ketosis can be induced through various dietary modifications. The ketogenic diet (KD) is characterized by low carbohydrate and high-fat intake, which prompts ketosis due to the availability of substrates. Additionally, ketone supplements, including ketone esters and ketone salts, effectively elevate ketone body levels [30].
KD/Ketone Supplements and Cardiovascular Health
One of the primary advantages of the KD in managing cardiovascular risk is its ability to promote weight loss. The consistent weight loss observed across various studies may be partly due to caloric restriction [66]. However, the KD has been shown to have a greater impact on weight loss than low-fat diets, with results that persist even after 1 year [67]. Interestingly, even with equal calorie consumption, a carbohydrate-restricted diet results in a higher resting energy expenditure than a low-fat diet [68]. This observation is corroborated by animal research, which indicates that the KD can increase energy expenditure without increasing caloric intake [69]. Concurrently, the KD is associated with a marked decrease in fasting glucose and HbA1c levels compared to other diets [70]. Therefore, the KD has demonstrated significant benefits in the metabolic management of patients with obesity and diabetes [71].
The KD appears to have a negative impact on the lipid profiles of rodents; however, in humans, the opposite is true, with the KD generally having a beneficial effect [71]. This discrepancy may be due to the high saturated fat content in the diets of animal models, while the human version of the KD typically contains more unsaturated fats. Most randomized controlled trials have demonstrated a significant decrease in total cholesterol and triglyceride levels, along with an increase in HDL [66]. Although some studies have noted a rise in LDL levels, which has prompted skepticism about the benefits of the KD, these findings are inconsistent and do not seem to be associated with adverse cardiovascular outcomes [66]. Similarly to IF, the KD influences the sympathetic nervous system and the RAAS, contributing to the regulation of blood pressure. Various studies have documented a notable reduction in blood pressure following adherence to the KD [66, 72].
Finally, the KD has anti-inflammatory and antioxidant effects, which are primarily attributed to the increase in β-OHB levels. This elevation in β-OHB, in turn, influences the NLRP3 inflammasome and the balance of oxidative stress. Additionally, the restriction of carbohydrates and the high concentration of omega-3 fatty acids may also contribute to the anti-inflammatory effects of the KD [66]. Similarly, the effect of the KD on endothelial function is associated with the impact of β-OHB, as previously described (Fig. 1).
Ketone salts and ketone esters represent alternative methods for elevating β-OHB levels. Clinical trials have demonstrated their efficacy in improving glycemic and lipid control, as well as enhancing myocardial blood flow [30]. Notably, in a study involving heart failure patients, a ketone supplement prompted a significant, dose-dependent increase in cardiac output [73].
KD/Ketone Supplements and Bladder Health
The KD was utilized to treat urinary infections long before the advent of antibiotics. In 1933, Fuller reported a high success rate of the KD in treating these infections, attributing the effectiveness to the presence of β-OHB in the acidic urine [74]. The KD has also been investigated as a therapeutic option in other areas of urology. For example, it has been shown to be effective in improving stress urinary incontinence in obese women, primarily through the control of risk factors [75]. Additionally, in an animal model of BPH, the KD significantly reduced prostate size and markers of oxidative stress [76]. Similarly, the KD has been found to significantly alleviate symptoms in patients with male accessory gland inflammation and is associated with an increased rate of α-blocker discontinuation [77]. Further research is necessary to fully understand the effects of the KD and ketone supplements on LUTS stemming from various etiologies. Nevertheless, the aforementioned effects on the cardiovascular control of MS components are promising for the treatment of LUTS (Fig. 1).
SODIUM-GLUCOSE COTRANSPORTER 2 INHIBITORS
Sodium-glucose cotransporter 2 inhibitors (SGLT2i) are a class of antidiabetic drugs that function by inhibiting glucose reabsorption in the proximal tubule, thereby enhancing glucose excretion in the urine. This leads to reduced plasma glucose levels, which in turn decreases the insulin/glucagon ratio and stimulates ketogenesis, causing an elevation in β-OHB. Following long-term SGLT2i treatment, β-OHB levels can increase by up to 78% [78].
SGLT2i and Cardiovascular Health
SGLT2i have a broad spectrum of actions that are beneficial to cardiovascular health, which helps to explain their remarkable outcomes in heart failure trials. In addition to improving glycemic control, SGLT2i contribute to weight loss, reduction in blood pressure, better lipid profile management, and decreased levels of inflammation and oxidative stress [79]. They also have direct subcellular effects that support cardiac ion homeostasis [79]. However, it is thought that their metabolic actions are of greatest significance. The “thrifty substrate” hypothesis suggests that SGLT2i shift the body’s energy source from carbohydrates to lipids. This shift leads to an increase in β-OHB, providing the heart with an alternative and advantageous fuel source, as previously described [80]. Consequently, it is an intriguing proposition that β-OHB may act as a mediator for the effects of SGLT2i. Furthermore, the role of SGLT2i in cardiac remodeling could be partially attributed to β-OHB’s inhibition of class I histone deacetylases and the NLRP3 inflammasome [78].
SGLT2i and Bladder Health
SGLT2i treatment has diuretic effect, which increases urine volume. This effect may be problematic and potentially aggravate LUTS. In fact, some studies report an increased daytime frequency and nocturia associated with the beginning of the treatment [81, 82]. However, the diuretic effect of SGLT2i is transitory because a new hemodynamic, renal and neurohumoral adaptation is established [83] and the distal nephron compensates for the lack of sodium absorption in the proximal tubule [84]. Therefore, it remains unknown whether the increased LUTS reported with the beginning of SGTLT2i treatment persist with the long-term use of the drug. Moreover, given the similarities between heart failure and bladder myogenic dysfunction pathophysiology, SGLT2i long-term use may prove to be valuable in the treatment of LUTS arising from bladder dysfunction [85]. SGLT2i counteract ischemia, inflammation, oxidative stress, apoptosis and fibrosis which are all pathological processes of bladder damage [85]. Additionally, the SGLT2i induced increase of β-OHB may also exert actions over the urological system, as already discussed previously (Fig. 1). However, further studies are needed to better characterize the role of SGLT2i in LUTS and to what extent ketogenesis enhancement is involved in the molecular pathways.
KETOACIDOSIS RISK
Rising the circulating levels of ketone bodies increases the risk o ketoacidosis. This is a state where the buffering capacity of ketone acids has been outreached and therefore the blood pH drops [25]. All the above-mentioned interventions (intermittent fasting, ketogenic diet/supplements and SGLT2i treatment) carry the potential risk of ketoacidosis induction. IF and KD prescribed by a trained physician have very low risk of ketoacidosis [25]. However, there are reports of self-administered regimens of IF and KD resulting in ketoacidosis [86-88]. Furthermore, SGTLT2i may induce an euglycemic ketoacidosis, not only because of ketone bodies increase but also due to volume depletion [84]. Once more, this is a predictable and preventable complication that physicians should be aware before starting this treatment [89].
CONCLUSIONS
Ketogenesis is a metabolic process that offers benefits for heart and bladder health by managing components of MS. β-OHB appears to be a crucial molecule in the involved pathways. Interventions that elevate serum β-OHB levels—such as fasting, a KD, ketone supplementation, and SGLT2i—have shown significant cardiovascular advantages and offer promising prospects for treating bladder dysfunction associated with MS.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTION STATEMENT
·Conceptualization: GFC, TAL, AC
·Writing - original draft: GFC, JO, IVB, IC, EAS, CBS, SMS, TAL, AC
·Writing - review & editing: GFC, JO, IVB, IC, EAS, CBS, SMS, TAL, AC
