 |
 |


- Search
| Int Neurourol J > Volume 28(1); 2024 > Article |
|
ABSTRACT
To assess the effectiveness and safety of various techniques of pudendal nerve neurolysis (PNN) in patients with pudendal nerve entrapment (PNE). A comprehensive literature search was conducted on May 20th, 2023, using Scopus, PubMed, and Embase databases. Only studies in English involving adults were accepted, while meeting abstracts and preclinical studies were excluded. A total of 34 papers were included. Transperineal PNN emerged as a promising technique, demonstrating significant potential in alleviating pain, restoring erectile function in males, and improving the resolution of urinary stress incontinence in females. Furthermore, the bilateral approach consistently yielded positive outcomes in addressing urinary symptoms. The transgluteal technique appeared particularly suitable for cases of posterior PNE, situated between the sacrospinous ligament and the lesser sciatic foramen. A progressive amelioration of painful symptoms was observed during follow-up. Minimally invasive PNN is evolving and enables decompression along the entire proximal tract up to the Alcock canal, minimizing the risk of comorbidities. In addition to reducing pudendal neuralgia, robot-assisted and laparoscopic approaches determined a reduction in lower urinary tract symptoms and an improvement in erectile function, though further studies are required to corroborate these findings. PNN emerges as an effective treatment for PNE with minimal morbidity. Therefore, PNN should be tailored according to the site of PNE to enhance functional outcomes and improve patient quality of life.
Pudendal neuralgia is a condition characterized by persistent pain originating from the pudendal nerve (PN) territory. According to the International Pudendal Neuropathy Group, its incidence is estimated at approximately 1 case per 100,000 individuals in the general population [1]. The multifaceted etiological underpinnings of pudendal neuralgia encompass a range of factors, including direct or chronic traumatic events, viral infections, or iatrogenic etiology [2]. Pudendal neuralgia typically involves the entrapment of a nerve within a tunnel-like anatomical structure, potentially leading to compression of the nerve itself or its branches [3]. Consequently, the clinical presentation varies on the specific affected area [4]. Due to the absence of definitive radiological or electrophysiological tests, the diagnosing of pudendal nerve entrapment (PNE) relies on clinical assessment and exclusion of other diseases. Currently, diagnostic criteria align with the 5 criteria of the Nantes classification [5].
Beyond the time-intensive diagnostic phase, effective resolution of the condition may necessitate additional time investment. Initial treatment primarily consists of pharmacological therapy, which plays a significant role in reducing the incidence and intensity of pain [6]. Pharmacological therapy is supplemented by behavioral and physical interventions, such as myofascial relaxation and tension alleviation, addressing the strain exerted on the PN by the internal obturator muscle [7]. Additionally, localized anesthetic injections can provide temporary analgesia by targeting the precise site of entrapment [8]. However, for sustained results, nerve decompression emerges as the primary option for achieving long-lasting benefits. As per the current European Association of Urology guidelines, nerve decompression is recommended in cases of nerve entrapment or injury, particularly for patients with pain persisting for fewer than 6 years [9]. Various approaches have been reported, encompassing transgluteal, transperineal, and minimally invasive approaches. Nevertheless, none of them provide a complete release of the entire nerve, and each technique comes with its own set of advantages and limitations. Therefore, the primary objective of this systematic review is to assess the outcomes of the various techniques for pudendal nerve neurolysis (PNN) as described in the literature. We also describe briefly each surgical technique.
A broad literature search was performed on May 20th, 2023, using PubMed, Scopus, and Embase using the following terms and Boolean operators: (release OR neurolysis OR decompression) AND (pudendal) AND (entrapment OR neuralgia OR nerve OR injury).
The PICOS model (Patient Intervention Comparison Outcome Study type) was used to frame and respond to the clinical question; P: patients with PNE; I: transgluteal, transperineal or minimally invasive PNN; C: other interventions or none; O: Resolution or improvements in pudendal neuralgia, or urogenital symptoms, and perioperative complications; S: retrospective and prospective studies; case reports.
Only English studies were accepted. Preclinical and animal studies were excluded, as were review articles, letters to the editor, and meeting abstracts. Two independent authors screened all retrieved studies using Covidence Systematic Review Management (Veritas Health Innovation, Melbourne, Australia). A third author resolved conflicts.
The literature search yielded a total of 723 papers. After automatically removing 363 duplicates, we screened the remaining 363 papers based on titles and abstracts. Among these, 293 papers were found irrelevant to the purpose of this review and were excluded. Finally, 67 full-text papers underwent further screening for appropriateness, leading to the exclusion of 33 additional papers. Finally, 30 papers met the inclusion criteria and were included [10-39]. Fig. 1 shows the diagram of the literature screening.
Among the included studies, 11 were retrospective [12-17,22,26,29,36,38], 11 were prospective [10,18,19,21,23,28,31-35], 7 were case reports [11,20,25,27,30,37,39], and one was a randomized trial [24]. The surgical techniques utilized exhibited variation, encompassing transperineal open surgery in 8 studies [10-17], transgluteal in 8 studies [18-25], and the remainder adopting minimally invasive approaches, which comprised 12 laparoscopic [26-37] and 2 robot-assisted [38,39] methods. Table 1 shows the characteristics of the included papers.
The transperineal approach, initially pioneered by Shafik [40], has emerged as a option suitable for a highly specific cohort of individuals diagnosed with distal PNE [10]. However, the ultimate selection of the surgical approach remains at the discretion of the surgeon [11]. This surgical technique implies a lithotomy position with patients under either general or local anesthesia [12]. Hruby et al. [10] described a 3-cm vertical incision laterally adjacent to the scrotum, near the origin of the scrotum. Shafik [40] undertook a vertical para-anal incision positioned 2 cm lateral to the anal orifice. During access to the ischiorectal fossa, identifying a dorsal branch variant traversing the Alcock canal is crucial. Then, focus shifted to tracing the course of inferior rectal branch of the PN from lateral to median across the ischiorectal fossa. This branch was gently stretched laterally to its origin from the PN, using the hooked index finger. Subsequently, the overlying fascia enshrouding the pudendal canal was incised along its complete length, effectively emancipating the nerve within the ischiorectal fossa. Beco et al. [17] also incorporated endoscopy into the procedure, with the aim of precisely identifying local anatomical landmarks during specific key steps.
Amongst patients subjected to the transperineal technique, no instances of operative or postoperative complications were documented [12-15]. Nevertheless, paramount attention during surgery was directed towards the release of the ischiocavernosus muscle to prevent inadvertent injury to the corpora cavernosa and to ensure complete liberation of fibrous attachments [10].
In a study conducted by Shafik, the effects of bilateral transperineal PNN were evaluated in 12 women affected by stress urinary incontinence (SUI) and manifestations of pudendal PNE. Over a mean follow-up period of 17.7 months, SUI exhibited marked improvement within 5 months for 11 patients (92%). Notably, 6 women achieved complete continence, while the remaining 5 displayed varying degrees of improvement. Sensory enhancements preceded motor improvements, occurring at 3–4 months postoperatively for sensory manifestations and after 4–5 months for motor symptoms [13].
Shafik [14] documented the functional outcomes among 7 men afflicted with erectile dysfunction (ED) alongside urinary urgency or hypoesthesia/anesthesia in the genital area. After a follow-up of 19.6 months, complete resolution of ED was achieved in 6 patients (85%), and urgency subsided in 5 out of 7 patients (71%) [14].
In a retrospective study by Beco et al. [16], 74 symptomatic women with perineal pain, and urinary incontinence underwent bilateral PNN. Within the cohort of 26 patients afflicted by perineal pain, 18 patients completed at least 12-month follow-up. Among these, a 61.1% cure rate and a 77.7% improvement rate were observed, with an average follow-up duration of 22.2 months. For urinary incontinence, which was exclusive in 5 patients, a 60% cure rate was noted over an average follow-up period of 18.5 months. Given the bilateral innervation of urinary sphincter, bilateral decompression emerges as a logical strategy for individuals presenting with urinary symptoms [16].
In a study by Klifto and Dellon [12], involving 5 patients with compromised penile sensation or painful penile episodes resulting from injury to the dorsal branch of the PN, recovery was evaluated over an average follow-up of 57 weeks. Notably, complete recovery of erogenous sensibility was achieved in 6 patients (83%), while 2 (67%) regained normal erections. Additionally, half of them experienced complete relief from ejaculatory pain and regained ejaculation function.
Ayik et al. [11] echoed these findings in a case report involving a patient with persistent perineal burning sensation and erectile discomfort persisting from a pelvic fracture and urethral injury sustained 27 years prior. After PNN, significant improvement in erectile function was documented through the International Index of Erectile Function (IIEF-5), a decrease in perineal and erection pain, and intercourse-related pain as measured with the visual analogue scale (VAS).
A prospective study by Hruby et al. [10] showcased functional nerve enhancement in all 5 patients afflicted by penile hyposensitivity and dysesthesia. Furthermore, resolution of ED was evident in 2 patients previously affected. Lastly, Klifto and Dellon [15], in a 2020 study, delved into the rare condition of persistent genital arousal disorder (PGAD) in women, attributing it to minimal dorsal branch compression of the PN. PNN was found to effectually alleviate pain, with 7 out of 8 patients experiencing complete resolution of arousal symptoms and 6 out of 7 reporting pain mitigation after a follow-up exceeding 26 weeks post-surgery. Symptomatic improvement demonstrated a positive correlation with symptom duration.
Finally, Beco et al. [17] presented the results of a study involving 113 patients with PNE, with a mean follow-up duration of 25.4 months. The endoscopic PNN demonstrated not only significant improvements in neurological pain and sensitivity (100% vs. 40.9% and 75.8% vs. 30.8%, respectively, both P<0.0001) but also in lower urinary tract symptoms (LUTS), such as nocturia and dysuria. Additionally, improvements were observed in urinary incontinence. Furthermore, the efficacy extended to the realm of sexual function, with a reduction in the rates of dyspareunia and PGAD, although not for ED.
The transgluteal open surgical technique for PNN, as pioneered by Robert et al. [41], entails positioning the patient in a prone jack-knife position under general anesthesia. This approach has been consistently adopted across various medical centers without variations [18-25].
The surgeon initiates bilateral gluteal incisions along a transverse line originating from the apex of the coccyx, with an oblique orientation aligned to the fibers of the gluteus maximus muscle. The muscle fibers are meticulously dissected and separated from the sacrotuberous ligament (STL), exposing the pudendal neurovascular bundle. A retractor is employed to hold the ischio-rectal fat medially, establishing access to the pudendal canal. A manual nerve release is performed, and in specific cases where the obturator fascia is thick or the falciform process presents a risk, incisions are made in these anatomical structures. To enable the frontal transposition of the nerve to the ischial spine, the sacrospinous ligament (SSL) is incised. Meticulous attention is required during the neurolysis and transposition of the PN to prevent neuronal damage. Popeney et al. [23] observed a relevant numbness and joint dysfunction rate in their case series of 58 patients. However, these complications were limited in scope and did not pose significant threats.
The transgluteal open approach for PN entrapment is generally considered a safe technique. Multiple studies have reported its overall safety, with some even indicating no complications [18,20,21,24,25]. Filler [19], in a study involving 200 patients treated using either open transgluteal or endoscopic approaches, documented only the occurrence of a deep hematoma 4 days postsurgery, requiring surgical intervention.
In a prospective study by Dellon et al. [18], encompassing 55 patients undergoing surgery for PNE, the selected surgical approach was transgluteal for cases involving rectal pain and transperineal for those without rectal pain. Outcomes were comparable across genders, anterior and posterior approaches. Notably, successful outcomes were linked to the surgeon’s learning curve, progressively improving over the study’s three-year span. Remarkably, 86% of patients achieved excellent results, while 14% attained good results.
Popeney et al. [23] outlined a diagnostic protocol for PNE and assessed clinical responses to surgical decompression within a prospective study involving 58 patients. The transgluteal surgical approach was adopted, primarily for chronic intractable neuropathic perineal pain. Through a preoperative to 12-month postoperative survey comparison, 60% of patients exhibited positive responses, classifying them as responders, while the remaining 40% were nonresponders.
Filler [19] conducted a prospective evaluation of 200 patients, utilizing advanced diagnostic technologies to differentiate PNE subtypes based on distinct entrapment sites. Patients underwent either open transgluteal or endoscopic approaches. The application of minimally invasive surgical techniques tailored to each subtype yielded favorable outcomes, with 87% experiencing significant improvement. Notably, most patients observed improvements within a month post-surgery, with progressive enhancements extending up to 1 year.
The transgluteal approach’s efficacy extends to bilateral PNE cases. Luther and Castellanos [20] presented a case report of a 39-year-old male with genital numbness, ED, and urinary frequency due to PNE. Following unilateral left transgluteal PNN a subsequent recovery of sensation in the left pelvic region occurred. To fully restore sensitivity, a right transgluteal PNN was necessary, leading to regained sexual function, and resolution of urinary frequency.
Mauillon et al. [21] conducted a prospective analysis of 12 patients to investigate the feasibility of predicting PNN efficacy using preoperative evaluations, including clinical symptoms, and electrophysiological tests, and PN blocks. A follow-up of up to 21 months revealed that 3 patients achieved complete relief, 1 demonstrated slight improvement, and 8 remained in pain. Surgical outcomes did not consistently correlate with preoperative clinical or electrophysiological data; however, a positive response to a nerve block emerged as a potential predictor of surgery success.
Ploteau et al. [22] described cases of 100 patients who underwent unilateral or bilateral nerve decompression through a transgluteal approach. The transgluteal approach’s benefit lies in its ability to provide continuous visual control over the extrapelvic trajectory of PN, allowing for early identification of potential variations. Notably, 70% of patients experienced significant improvement after surgery, while 30% continued to experience persistent pain.
The transgluteal approach efficacy was affirmed in its initial applications. Robert et al. [24] treated 400 patients for PNE since 1987 and conducted a randomized controlled trial. Sixteen patients were assigned to the surgery and control groups. Evaluations at 3 months, 1 year, and 4 years postsurgical decompression revealed markedly superior pain improvement in the surgery group (50% vs. 6% at 3 months, 71% vs. 13% at 1 year).
The first technical depiction of laparoscopic approach to PN was provided by Possover [26] in 2009. In their study, the authors proposed the laparoscopic decompression of the endopelvic section of the sciatic and PN in females to alleviate chronic pain symptoms in the perineal, perianal, or lower abdominal regions.
This technique involved transecting the SSL to facilitate PN decompression. For patients without nerve lesions or those with systemic neurological conditions (e.g., multiple sclerosis), a neuromodulator was concurrently implanted as part of the LION (laparoscopic implantation of neuroprosthesis) procedure, with the aim to modulate afferent neuronal transmission from S2–5 sacral routes. A similar technique was also documented in the case report by Moncada et al. [27].
In the urological field, the first report of laparoscopic PN release was presented and meticulously delineated by Erdogru et al. [28] as the “Istanbul technique.” After the peritoneal incision, the internal iliac artery and vein are identified, revealing the arcus tendinous fascia of the pelvis upon medial retraction and exposing the SSL. The dissection continued until the proximal entrance of Alcock canal. Coccygeus muscle fibers were progressively incised, revealing the pudendal elements, which were then transposed and shielded by an omental flap. The latter was affixed to the lower part of the arcus tendinous with sutures. The authors successfully treated a series of 25 patients, but this technique determined extended surgical durations (up to 300 minutes) and a notable incidence of postoperative complications. These complications included 2 cases of localized numbness in the PN area (Clavien-Dindo classification grade [CD] I) and 4 cases of CD II complications.
To nuance some technical aspects of this technique, Bollens provided a comprehensive anatomical depiction and standardization of the laparoscopic approach [29-32]. Briefly, the peritoneum is incised laterally to the umbilical artery, forming a peritoneal window between the fatty tissue covering the iliac vessels and the bladder. This incision extended to the obturator space, where the obturator vein was pinpointed. The fatty tissue is dissected medially to the vein and swept to expose the underlying internal obturator muscle. This muscle’s identification leads to the arcus tendinous fascia pelvis, followed by its posterior exposure to reveal the sciatic spine and the upper segment of the STL. The ligament is meticulously transected, and the pudendal elements are isolated and transposed to the entrance of Alcock canal, culminating in the incision of the proximal end of Alcock canal roof for complete PN release. Unlike the “Istanbul technique,” these authors omitted the omental flap surgical step to streamline surgical time and improve reproducibility.
This technique appeared feasible and reproducible in author’s experience of more than 235 cases [29]. The authors reported a mean operation time of 33.9 minutes and only 1 intraoperative complication (1 case of bleeding from pudendal artery lesion, managed with laparoscopic suture). Postoperative complications were mainly of CD I such as genital numbness in 36 of 235 (15%); Urinary retention in 1 of 235 (0.4%) and transient fecal incontinence in 2 of 235 (0.8%), and only one case of CD II—an infected lymphocele (0.4%), necessitating prolonged antibiotic treatment.
More recently, a novel endoscopic minimally invasive technique called ENTRAMI (endoscopic transgluteal minimal-invasive) was introduced to address PN and inferior cluneal nerve entrapment. This technique involved a transgluteal approach via 2 small incisions of 3 mm and 5 mm, granting access to the PN along its entire course, including potential entrapment sites. This approach aimed to minimize surgical trauma [32-35].
Regarding the robotic approach, after a few initial cases of PNN, initially performed and described by Rey and Oderda [36], a significant paper detailing more substantial outcomes was reported by Giulioni et al. [37]. The steps of this surgical technique closely mirrored those presented by Bollens et al. [29]. The complication rate was low, with only one patient reporting postoperative pain that was alleviated with analgesics (CD I).
In the case series by Possover [26], retrospectively evaluating 134 patients suffering from anogenital pain, of the 18 patients with PNE, at a mean follow-up of 21 months, the laparoscopic decompression was deemed successful in 15, with a significant improvement in pain (mean decrease in the preoperative VAS score from 9.1 to 1.6).
Efficacy data in terms of pain relief presented in the work by Erdogru et al. [28] were also promising. A statistically significant reduction in VAS scores was seen in all patients, and this rate was 81% in the subcohort of 16 patients followed-up for >6 months; similarly, the quality-of-life scores significantly improved postoperatively. According to the authors, key aspects for these good results could be attributed to the omental flap protection performed during the procedure.
In the study by Bollens et al. [29], with regards to efficacy, for the subcohort of 32 patients who were prospectively followed for a mean follow-up of 6.2 months, the intervention was effective on most of the symptoms of PNE. The pain in the PN territory was significantly improved in all patients at 3 months (mean VAS score 6.8 vs. 2.2). On the contrary, urinary symptoms improvement was less evident, with no improvement in women with SUI or overactive bladder symptoms (P>0.05), whereas the best results were seen in young male patients with LUTS (mean Urinary Symptom Profile dysuria domain score 4.2 vs. 1.6). Similar efficacious data on urinary outcomes were also reported by the same group in a prospective case series including 5 young males suffering from LUTS who underwent laparoscopic PNN. Specifically, a significant improvement in International Prostate Symptom Score and maximal flow on uroflowmetry was observed 3 months after surgery (18 mL/sec vs. 8 mL/sec, and 12 mL/sec vs. 18 mL/sec, respectively) [31]. With regards to sexual symptoms, for men suffering from mild to moderate ED, there was a rapid response within 1 month after surgery (mean IIEF-5 score 15.2 vs. 19.3), mainly due to pudendal artery release [29]. Further supportive literature was provided by the same group of authors, who showed significant and rapid amelioration of ED after laparoscopic decompression of pudendal artery and nerve in young healthy males presenting with ED not responsive to medical therapy. In a first case report by Maggi et al. [30], in a 32-year-old man with a complaint of ED, pudendal compression was the suspected etiology, and PN and artery decompression was laparoscopically performed. As early as 1 month after the operation, there was an improvement in the erectile function, which persisted at the last follow-up of 8 months (IIEF-5 score 5.0 vs. 18.0). In line with this first experience, a prospective case series enrolling 5 patients, laparoscopic treatment of PN and artery entrapment improved ED (mean IIEF-5 score 13.0 vs. 18.8 at 3-month follow-up) [32].
Efficacy outcome of laparoscopic decompression approach for the pudendal and sciatic nerves to surgically treat pudendal neuralgia was recently presented also in the paper by Kale et al. [39]. In this case report, 1 day after the intervention, pain dramatically decreased, and at 3- and 6-month follow-up examinations completely resolved (VAS score 10 vs. 0), as well as urinary frequency and nocturia.
Efficacy data on pain relief of the robotic surgical approach were provided by the study by Giulioni et al. [37], and explored at 3 and 6 months postoperatively, according to numeric pain rating scale (NPRS), which significantly improved. Specifically, NPRS score progressively reduced (8 vs. 6 and 6 vs. 4 after three and 6 months from surgery, respectively). Furthermore, a negative relationship between the duration of pain and the improvement in NPRS score was noted (Pearson correlation coefficient=-0.81).
The present study has some limitations, starting from the presence of a single randomized trial. Secondly, most of the included studies were single center, small retrospective series which preclude us to give some clear take-home messages based on high quality evidence. Thirdly, included studies were published by referral centers and outcomes can be different in nonexperienced hands. Finally, there is a lacking of data on long-term outcomes following PNN having most studies a follow-up no longer than 2 years. However, the strength of our study relies on the “state of the art” description of surgical outcomes of patients suffering from urinary and sexual dysfunctions due to PNE and we believe that this review can be useful for any medical personnel involved in the management of these patients.
In this systematic review, we comprehensively assessed and described available surgical technique for PNN in patients suffering from PNE, highlighting each advantage and disadvantage. We found that surgical decompression of PNE is a safe procedure with minimal postoperative morbidity. Yet, PNN was associated with improvement in urinary and sexual dysfunction, and perineal/genital pain. PNN and particularly minimally invasive approaches should be offered in patients with PNE symptoms refractory to medical treatments.
REFERENCES
1. Hibner M, Desai N, Robertson LJ, Nour M. Pudendal neuralgia. J Minim Invasive Gynecol 2010;17:148-53. PMID: 20071246


2. Soon-Sutton TL, Feloney MP, Antolak S. Pudendal neuralgia. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-.
3. Kaur J, Singh P. Pudendal nerve entrapment syndrome. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-.
4. Martin R, Martin HD, Kivlan BR. Nerve entrapment in the hip region: current concepts review. Int J Sports Phys Ther 2017;12:1163-73. PMID: 29234567



5. Labat JJ, Riant T, Robert R, Amarenco G, Lefaucheur JP, Rigaud J. Diagnostic criteria for pudendal neuralgia by pudendal nerve entrapment (Nantes criteria). Neurourol Urodyn 2008;27:306-10. PMID: 17828787


6. Carroll I, Hah J, Mackey S, Ottestad E, Kong JT, Lahidji S, et al. Perioperative interventions to reduce chronic postsurgical pain. J Reconstr Microsurg 2013;29:213-22. PMID: 23463498



8. Peng PW, Tumber PS. Ultrasound-guided interventional procedures for patients with chronic pelvic pain - a description of techniques and review of literature. Pain Physician 2008;11:215-24. PMID: 18354713


9. Engeler D, Baranowski AP, Berghmans B, Borovicka J, Cottrell AM, Dinis-Oliveira P, et al. EAU guidelines on chronic pelvic pain. Arnhem (Netherlands): European Association of Urology; 2023.
10. Hruby S, Dellon L, Ebmer J, Höltl W, Aszmann OC. Sensory recovery after decompression of the distal pudendal nerve: anatomical review and quantitative neurosensory data of a prospective clinical study. Microsurgery 2009;29:270-4. PMID: 19274651


11. Ayık Ö, Kozanoğlu E, Önol Y, Durmaz H. Post-traumatic double crush pudendal nerve entrapment syndrome after fracture of the pelvis: a case report. Acta Orthop Traumatol Turc 2021;55:277-80. PMID: 34100371



12. Klifto KM, Dellon AL. Neurolysis of the dorsal nerve to the penis to restore function after trauma. Ann Plast Surg 2020;84:307-11. PMID: 31904648


13. Shafik A. Pudendal canal decompression in the treatment of urinary stress incontinence. Int Urogynecology J 1994;5:215-20. 

14. Shafik A. Pudendal canal decompression in the treatment of erectile dysfunction. Arch Androl 1994;32:141-9. PMID: 8166577


15. Klifto K, Dellon AL. Persistent genital arousal disorder: treatment by neurolysis of dorsal branch of pudendal nerve. Microsurgery 2020;40:160-6. PMID: 31025770



16. Beco J, Climov D, Bex M. Pudendal nerve decompression in perineology: a case series. BMC Surg 2004;4:15. PMID: 15516268




17. Beco J, Seidel L, Albert A. Endoscopic transperineal pudendal nerve decompression: operative pudendoscopy. Surg Endosc 2018;32:3720-31. PMID: 29796819



18. Dellon AL, Coady D, Harris D. Pelvic pain of pudendal nerve origin: surgical outcomes and learning curve lessons. J Reconstr Microsurg 2015;31:283-90. PMID: 25629207


19. Filler AG. Diagnosis and treatment of pudendal nerve entrapment syndrome subtypes: imaging, injections, and minimal access surgery. Neurosurg Focus 2009;26:E9. PMID: 19323602



20. Luther RD 3rd, Castellanos ME. Successful treatment of penile numbness and erectile dysfunction resulting from pudendal nerve entrapment. Urology 2019;134:228-31. PMID: 31560912


21. Mauillon J, Thoumas D, Leroi AM, Freger P, Michot F, Denis P. Results of pudendal nerve neurolysis-transposition in twelve patients suffering from pudendal neuralgia. Dis Colon Rectum 1999;42:186-92. PMID: 10211494


22. Ploteau S, Perrouin-Verbe MA, Labat JJ, Riant T, Levesque A, Robert R. Anatomical variants of the pudendal nerve observed during a transgluteal surgical approach in a population of patients with pudendal neuralgia. Pain Physician 2017;20:E137-43. PMID: 28072805


23. Popeney C, Ansell V, Renney K. Pudendal entrapment as an etiology of chronic perineal pain: Diagnosis and treatment. Neurourol Urodyn 2007;26:820-7. Erratum in: Neurourol Urodyn 2008;27:360.
24. Robert R, Labat JJ, Bensignor M, Glemain P, Deschamps C, Raoul S, et al. Decompression and transposition of the pudendal nerve in pudendal neuralgia: a randomized controlled trial and long-term evaluation. Eur Urol 2005;47:403-8. PMID: 15716208


25. Uruc V, Ozden R, Kalaci A, Dogramaci Y, Başarslan SK. Unilateral complete ossification of sacrotuberous ligament: an unusual cause of pudendal nerve entrapment syndrome. Neurosurg Q 2016;26:185-7.
26. Possover M. Laparoscopic management of endopelvic etiologies of pudendal pain in 134 consecutive patients. J Urol 2009;181:1732-6. PMID: 19233408


27. Moncada E, de San Ildefonso A, Flores E, Garrido L, Cano-Valderrama O, Vigorita V, et al. Right laparoscopic pudendal release+ neurostimulator prosthesis (LION procedure) in pudendal neuralgia. Colorectal Dis 2022;24:1243-44. PMID: 35575432

28. Erdogru T, Avci E, Akand M. Laparoscopic pudendal nerve decompression and transposition combined with omental flap protection of the nerve (Istanbul technique): technical description and feasibility analysis. Surg Endosc 2014;28:925-32. PMID: 24149853



29. Bollens R, Mjaess G, Sarkis J, Chemaly AK, Nemr E, Daher K, et al. Laparoscopic transperitoneal pudendal nerve and artery release for pudendal entrapment syndrome. Surg Endosc 2021;35:6031-8. PMID: 33048235



30. Maggi M, Pirola GM, Absil F, De Plaen E, Mosca A, Salciccia S, et al. Erectile function recovery after laparoscopic decompression of pudendal artery and nerve: a documented case report. Cent European J Urol 2020;73:569-71. PMID: 33552586


31. Aoun F, Mjaess G, Akl B, Nassar D, Kallas Chemaly A, Haydar A, et al. Pudendal nerve release for lower urinary tract symptoms in young males. Low Urin Tract Symptoms 2021;13:286-90. PMID: 33283436



32. Aoun F, Mjaess G, Daher K, Sakr G, Chemaly AK, Salameh M, et al. Laparoscopic treatment of pudendal nerve and artery entrapment improves erectile dysfunction in healthy young males. Int J Impot Res 2021;33:1-5. PMID: 32366984



33. Possover M, Forman A. Pelvic neuralgias by neuro-vascular entrapment: anatomical findings in a series of 97 consecutive patients treated by laparoscopic nerve decompression. Pain Physician 2015;18:E1139-43. PMID: 26606029


34. Jottard K, Bruyninx L, Bonnet P, Mathieu N, De Wachter S. Pilot study: pudendal neuromodulation combined with pudendal nerve release in case of chronic perineal pain syndrome. The ENTRAMI technique: early results. Int Urogynecol J 2021;32:2765-70. PMID: 33048179



35. Jottard K, Bruyninx L, Bonnet P, De Wachter S. Endoscopic trans gluteal minimal-invasive approach for nerve liberation (ENTRAMI technique) in case of pudendal and/or cluneal neuralgia by entrapment: one-year follow-up. Neurourol Urodyn 2020;39:2003-7. PMID: 32678485



36. Rey D, Oderda M. The first case of robotic pudendal nerve decompression in pudendal nerve entrapment syndrome. J Laparoendosc Adv Surg Tech A 2015;25:319-22. PMID: 25790267


37. Giulioni C, Asimakopoulos AD, Annino F, Garelli G, Riviere J, Piechaud-Kressmann J, et al. First case-series of robot-assisted pudendal nerve release: technique and outcomes. Surg Endosc 2023;37:5708-13. PMID: 37208481



38. Lemos N, Sermer C, Fernandes G, Morgado-Ribeiro A, Rossos A, Zhao ZY, et al. Laparoscopic approach to refractory extraspinal sciatica and pudendal pain caused by intrapelvic nerve entrapment. Sci Rep 2021;11:10820. PMID: 34031480




39. Kale A, Basol G, Topcu AC, Gundogdu EC, Usta T, Demirhan R. Intrapelvic nerve entrapment syndrome caused by a variation of the intrapelvic piriformis muscle and abnormal varicose vessels: a case report. Int Neurourol J 2021;25:177-80. PMID: 33504131




40. Shafik A. Pudendal canal syndrome. Description of a new syndrome and its treatment. Report of 7 cases. CoIoproctology 1991;13:102-9.
41. Robert R, Labat JJ, Lehur PA, Glemain P, Armstrong O, Le Borgne J, et al. Réflexions cliniques, neurophysiologiques et thérapeutiques à partir de données anatomiques sur le nerf pudendal (honteux interne) lors de certaines algies périneales. Chirurgie 1989;115:515-20. PMID: 2637109

Fig. 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) 2009 flow diagram detailing the search strategy and identification of studies used in data synthesis.
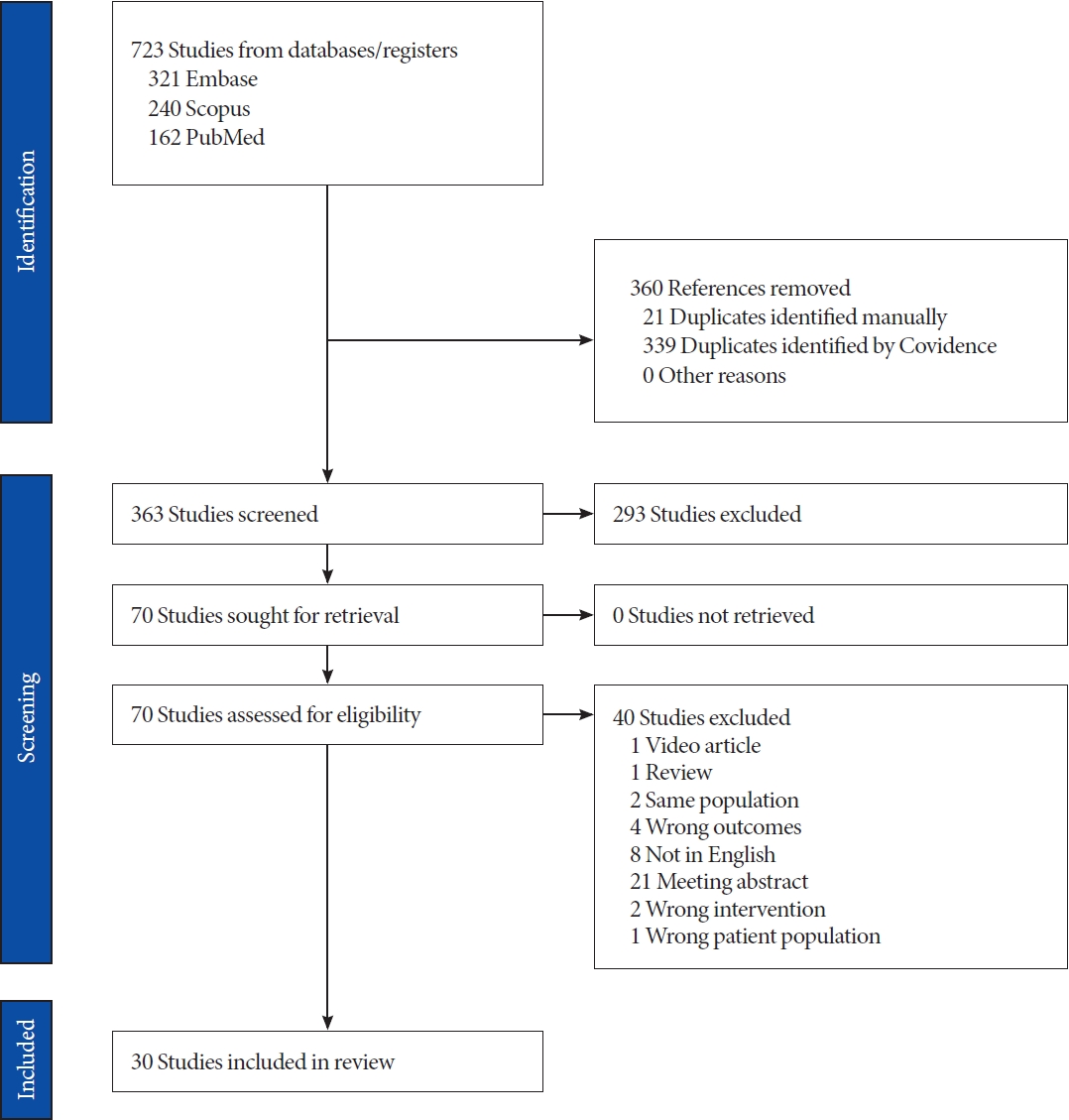
Table 1.
Studies concerning the various techniques of pudendal nerve neurolysis (PNN) and their impact on functional outcomes in patients affected by pudendal nerve entrapment (PNE)
| Study | Country | Type of study | No. of patients | Preoperative pain score | Associated disorder | Approach technique | Postoperative pain score | Postoperative complications | Follow-up (mo) | Final considerations |
|---|---|---|---|---|---|---|---|---|---|---|
| Hruby et al. (2009) [10] | Austria | Prospective | 5 | - | Penile dysesthesia, ED | Transperineal | - | None | 18 | PNN effectively resolves sensory impairments, as evidenced by Quantitative Sensory Testing, which can distinguish between proximal and distal nerve lesions |
| Ayık et al. (2021) [11] | Turkey | Case report | 1 | VAS: 8 | Urinary incontinence, ED | Transperineal | VAS: 3 | None | 12 | Selective decompression was correlated with reduction in pain and enhanced functional outcomes |
| Klifto and Dellon (2020) [12] | USA | Retrospective | 7 | - | ED and genital numbness | Transperineal | - | Numbness: 1 (14) | 14 | PNN resolved the erectile disorder and restore genital sensation in most cases |
| Shafik (1994) [13] | Egypt | Retrospective | 12 | - | SUI and genital numbness | Transperineal | - | None | 18 | PNN determined a resolution of SUI and genital anesthesia |
| Shafik (1994) [14] | Egypt | Retrospective | 7 | - | ED and genital numbness | Transperineal | - | None | 20 | An improvement of ED, with reduction in bulbocavernosus reflex latency and pudendal nerve terminal motor latency, occurred |
| Klifto and Dellon (2020) [15] | USA | Retrospective | 8 | - | PGAD | Transperineal | - | None | 6 | Bilateral PNN may be necessary to resolve PGAD |
| Beco et al. (2004) [16] | Belgium | Retrospective | 74 | - | Anal and/or urinary incontinence | Transperineal | - | Anemia: 1 (1.3) | 12 | Bilateral PNN resolved or improved and or urinary incontinence in most cases |
| Beco et al. (2018) [17] | Belgium | Retrospective | 113 | VAS: 7.2 | - | Transperineal+endoscopy | VAS: 4.5 | Wound infection: 1 (0.9) | 24 | The utilization of endoscopy allows the surgeon to thoroughly assess various stages of the PNN, ensuring optimal functional outcomes |
| Artery embolization for hemostasis: 1 (0.9) | ||||||||||
| Dellon et al. (2015) [18] | USA | Prospective | 55 | - | PGAD, rectal or iatrogenic pain | Transgluteal in case of rectal pain; transperineal in absence of rectal pain | - | - | 14 | A site-specific surgical approach favors an amelioration of urogenital disorders |
| Filler (2009) [19] | USA | Prospective | 200 | - | Disruption of sensation or function in the perineum, genitalia, or rectum | Transgluteal | - | Re-surgery for hemostasis: 1 (0.5) | 12 | After PNN, the genitalia numbness improved within the first month, with progressive enhancements extending up to one year |
| Luther and Castellanos (2019) [20] | USA | Case report | 1 | - | Numbness of the penis and erectile dysfunction | Bilateral transgluteal in 2 times | - | - | 24 | PNN demonstrated notable efficacy in resolving penile numbness |
| Mauillon et al. (1999) [21] | France | Prospective | 12 | - | Anal and/or genital pain | Transgluteal | - | - | 21 | A timely decompression is necessary to achieve satisfactory resolution of neuralgia |
| Ploteau et al. (2017) [22] | France | Retrospective | 100 | - | Pudendal pain | Transgluteal | - | - | - | This approach ensures optimal symptom resolution in PNE between the sacrospinous and sacrotuberous ligaments |
| Popeney et al. (2007) [23] | USA | Prospective | 58 | - | LUTS and sexual symptoms | Transgluteal | - | Numbness: 7 (12); sacroiliac joint dysfunction: 5 (8.6); transient urinary incontinence: 1 (1.2) | 12 | PNN resolved the urogenital disorders, with a low rate of morbidity |
| Robert et al. (2005) [24] | France | Randomized Controlled Trial | 16 | VAS: 8.3 | - | Transgluteal | VAS: 0 and <4 in 4 and 8 cases, respectively | None | 48 | PNN has a greater rate of neuralgia resolution than medical therapy |
| Uruc et al. (2016) [25] | Turkey | Case report | 1 | - | - | Transgluteal | - | - | 1 | PNN determined a tempestive relieve in pain after 1 month from surgery |
| Possover (2008) [26] | Switzerland | Retrospective | 18 | VAS: 8.9 | Genital dysesthesia | Laparoscopic transperitoneal+sacral plexus neuromodulation | VAS: 1.6 | - | 21 | PNN assisted by neuromodulation resulted in a significant reduction in pain levels |
| Moncada et al. (2022) [27] | Spain | Case report | 1 | - | Pudendal pain | Laparoscopic transperitoneal+sacral plexus neuromodulation | - | None | - | The laparoscopic technique ensured optimal visualization of the pudendal nerve, resulting in subsequent favorable outcomes |
| Erdogru et al. (2014) [28] | Turkey | Prospective | 27 | VAS: 5.6 | LUTS, constipation, and sexual dysfunction | Laparoscopic transperitoneal | VAS: 2 | Numbness: 1 (3.7), transient urinary retention: 1 (3.7), deep venous thrombosis: 1 (3.7), peroneal nerve paralysis: 1 (3.7) | 7 | This technique demonstrates a significantly high rate of pain relief, accompanied by enhanced safety attributed to omental flap interposition |
| Bollens et al. (2021) [29] | Belgium | Retrospective | 235 | VAS: 6.8 | LUTS, rectal symptoms, ED | Laparoscopic transperitoneal | VAS: 2.2 | Genital numbness: 36 (15); urinary retention: 1 (0.4); transient anal incontinence: 2 (0.8); lymphocele: 1 (0.4) | 24 | Laparoscopic release of pudendal nerve and artery determined an improvement of pain, erectile function and rectal symptoms |
| Maggi et al. (2020) [30] | Belgium | Case report | 1 | - | ED and genital numbness | Laparoscopic transperitoneal | - | None | 24 | PNN is an effective option for erectile function in case of PNE |
| Aoun et al. (2021) [31] | Belgium | Prospective | 5 | - | LUTS | Laparoscopic transperitoneal | - | - | 3 | Laparoscopic technique guaranteed an improvement of urinary flow and urological symptoms |
| Aoun et al. (2021) [32] | Belgium | Prospective | 5 | - | ED | Laparoscopic transperitoneal | - | Temporary genital numbness: 1 (20) | 3 | PNN may promptly restore erectile functionality in young male with PNE |
| Possover and Forman (2015) [33] | Switzerland | Prospective | 97 | - | - | Laparoscopic transperitoneal | - | None | 24 | Laparoscopic surgery guarantees a high success rate, minimizing the morbidities |
| Jottard et al. (2021) [34] | Belgium | Prospective | 16 | NPRS: 9.5 | - | Laparoscopic transperitoneal+pudendal neuromodulation | NPRS: 3.5 | None | 1 | The implementation of short-term pudendal neuromodulation was associated with a significant reduction in neuralgia just 1 month after surgery |
| Jottard et al. (2020) [35] | Belgium | Prospective | 15 | NPRS: 9 | - | Laparoscopic transperitoneal | NPRS: 5 | None | 12 | The transperitoneal approach significantly reduced pain sensation, with a high rate of good treatment response reported in most patients |
| Lemos et al. (2021) [38] | Brazil | Retrospective | 63 | - | Pain | Laparoscopic transperitoneal | NPRS: 2.7 | PN transection: 1 (1.5); ON tear: 1 (1.5) ureteral injury: 1 (1.5); GF neuropathy: 2 (3%); incisional hernia: 1 (1.5); rectovaginal fistula: 1 (1.5); Piriformis muscle adhesion to the sciatic nerve requiring a transgluteal neurolysis: 1 (1.5) | 12 | Laparoscopic PNN has been demonstrated as an effective and replicable surgical technique, necessitating prompt intervention to enhance the likelihood of success |
| Kale et al. (2021) [39] | Turkey | Case report | 1 | VAS: 10 | LUTS, dyspareunia, and hyperesthesia | Laparoscopic transgluteal | VAS: 0 | None | 6 | Laparoscopic PNN determined an amelioration of pain and sexual dysfunction |
| Rey and Oderda (2015) [36] | France | Case report | 1 | VAS: 8 | - | Robot-assisted transperitoneal | VAS: 2 | None | 6 | Robotic PNN presents a viable option, accompanied by numerous potential advantages associated with this platform |
| Giulioni et al. (2023) [37] | Italy | Retrospective | 32 | NPRS: 8 | - | Robot-assisted transperitoneal | NPRS: 6 | Postoperative pain requiring analgesics | 6 | A robotic approach could be considered a viable option for PNE, necessitating timely surgery, given the inverse correlation between the pain duration and the pain improvement |





