ŌĆó HIGHLIGHTS
- The study emphasizes the advantageous effects of exercise training for individuals with sepsis, as it sustains proinflammatory responses and boosts antioxidant activity.
- This suggests exercise could mitigate the harmful impacts of sepsis and enhance survival rates.
INTRODUCTION
The most common cause of death in critical care units is sepsis, also known as blood poisoning. Patients with sepsis face extended hospital stays and are approximately 8 times more likely to die during hospitalization than patients without sepsis [1]. Septic shock, a life-threatening form of sepsis, is characterized by a systemic inflammatory response, immune suppression, and multiple organ dysfunction. The incidence of sepsis is on the rise, with reports indicating 31.5 million cases annually and a mortality rate of 22.5% [2,3]. Deaths related to sepsis occur during both the early hyperdynamic phase and the late hypodynamic phase of the condition. Within the first 3 days, patients with sepsis experience considerable inflammation driven by proinflammatory cytokines, which is followed by a phase of immunosuppression mediated by anti-inflammatory cytokines [4]. Initially, patients display both proinflammatory and antiinflammatory responses; however, as the disease progresses, the production of proinflammatory cytokines diminishes, and the dominant response shifts from pro- to anti-inflammatory. As a result, many patients who survive the initial hyperdynamic phase enter a state of immunocompromise, which heightens their susceptibility to hemodynamic instability and markedly increases the risk of secondary infection [5].
Damaged cells can undergo cell death through either a programmed mechanism (apoptosis) or an unprogrammed process (necrosis). In addition, necroptosis, a regulated form of necrosis, has been recently identified. Necroptosis refers to programmed necrosis that exhibits similarities with extrinsic apoptosis but is morphologically distinct [6]. Although extrinsic apoptosis and necroptosis are triggered by the same signaling moleculesŌĆötumor necrosis factor (TNF) and Fas ligandŌĆöthe binding of these ligands activates only a single cell death pathway at a time. The pathway selected depends on whether the cleavage of caspase-8 or the phosphorylation of receptor-interacting serine/threonine-protein kinase 1 (RIP1) occurs [7]. During apoptosis, which is caspase-dependent, macrophages engulf and phagocytose the damaged cells. In contrast, cells undergoing caspase-independent necroptosis release their contents into the extracellular space. This release occurs through the phosphorylation of mixed lineage kinase domain-like protein (MLKL) and the formation of a necrosome complex, which can amplify the inflammatory response and augment tissue damage [7,8].
Endotoxins released by pathogens during sepsis trigger a proinflammatory response that recruits immune cells and promotes phagocytosis through the production of reactive oxygen species (ROS). These processes are energy-intensive, further increasing ROS production [9]. Although ROS represent important mediators in the elimination of pathogens, their prolonged presence can lead to the sustained expression of proinflammatory genes, potentially causing systemic inflammation and contributing to the lethal effects of sepsis [10]. Excessive ROS can also damage mitochondria and deplete energy stores, favoring the formation of necrosomes over apoptosomes and triggering the proinflammatory cell death pathway [11]. However, this proinflammatory state is rapidly balanced by a compensatory anti-inflammatory response that influences the immune system and encourages apoptosis [12]. Consequently, during sepsis, affected organs experience not only necrosis but also widespread apoptosis, leading to the generation of apoptotic bodies that are then phagocytosed by immune cells such as macrophages and dendritic cells. This immune response amplifies anti-inflammatory reactions, which in turn contributes to the loss of immune cells and the onset of sepsis-induced immunosuppression [5,13].
Septic shock impacts the brain, leading to potentially fatal conditions in patients with sepsis; these include heightened permeability of the blood-brain barrier, which results in sepsisassociated encephalopathy [14]. Sepsis-induced brain damage and neural inflammation particularly affect the hippocampus, a vulnerable region of the brain, as neural damage elevates proinflammatory cytokine levels in the affected area. This proinflammatory response draws a large influx of immune cells to the region, intensifying hippocampal inflammation and leading to neurodegeneration [15].
Because the brain relies on aerobic oxidative phosphorylation, it is highly susceptible to oxidative stress and necroptosis during sepsis [16]. Normally, redox balance is maintained by the production of antioxidants that scavenge toxic ROS and interrupt lipid peroxidation in cell membranes. These antioxidants also inhibit proinflammatory cytokine signaling and regulate inflammatory responses. Consequently, maintaining adequate antioxidant levels is essential to neutralize ROS and protect the host immune system [10]. In this context, physical exercise training is recognized as a powerful stimulus for eliciting both functional and morphological adaptations at the cellular level. These changes include improved mitochondrial function and increased antioxidative capacity [17]. The enhancement of mitochondrial function is particularly noteworthy, as it facilitates the generation of additional energy that can be utilized to combat invading pathogens and strengthen immune responses [10,18]. Although a concomitant increase in ROS production occurs during energy generation, the upregulation of antioxidant enzyme activity acts as a protective mechanism, safeguarding the host from the harmful effects of oxidative stress.
Recent animal studies have demonstrated that exercise training offers protective benefits against sepsis and can increase survival rates [19-21]. Osuru et al. [19] found that exercise training improved survival in rats following cecal ligation and puncture (CLP) by reducing inflammatory cytokines and mitigating the sepsis-induced suppression of key antioxidant defense components, such as superoxide dismutase (SOD) and nuclear factor erythroid 2-related factor 2 (Nrf2). Additionally, this exercise intervention effectively attenuated markers of oxidative and nitrosative stress after CLP. Exercise interventions have also been observed to lessen liver damage by boosting fatty acid oxidation, which in turn increases survival rates in mice treated with lipopolysaccharide (LPS) [20]. Similarly, another study reported that running on a treadmill decreased lung and distal organ damage, promoting improved survival rates in a CLP model. This benefit was linked to the restoration of a balance between inflammation and immune response [21].
Prior research has suggested that exercise may postpone the suppression of the immune system by modulating oxidative stress and its subsequent responses. However, the effect of exercise training on neural cell death, which is triggered by inflammatory responses, and its ability to curb the generation of ROS during sepsis remains unclear. In this study, we explored the effects of exercise training on sepsis by evaluating markers of apoptotic and necroptotic cell death, as well as ROS production, in the hippocampal neurons of mice. We utilized a CLP-induced sepsis model and measured these parameters 24 hours after CLP surgery. This time frame is critical because it is marked by a shift from hyperinflammation to immunosuppression, characterized by relatively low cytokine levels and high bacterial loads [22].
MATERIALS AND METHODS
Animals
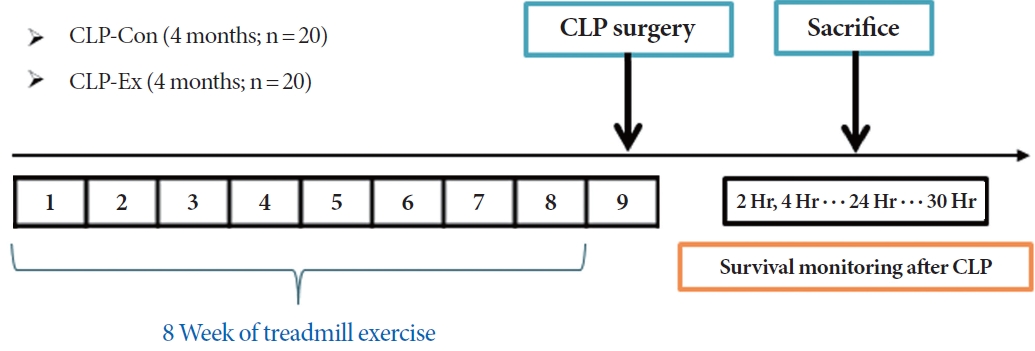
For this study, 40 male C57BL/6N mice, aged 4 months, were procured from Orient Bio Inc. (Seongnam, Korea). The mice were housed in pairs and had unrestricted access to food and water. They were kept in a pathogen-free animal care facility with controlled environmental conditions, including a 12-hour light/dark cycle, 50% humidity, and a temperature range of 20┬░CŌĆō23┬░C. The mice were divided into control (termed CLPCon; n=20) and experimental (CLP-Ex; n=20) groups, as illustrated in Fig. 1. The CLP-Ex mice were subjected to an 8-week exercise regimen on a rodent treadmill. Following this period, all animals from the CLP-Con and CLP-Ex groups underwent CLP surgery. After the procedure, the mice were further divided into 2 subsets: 10 mice from each group were sacrificed 24 hours after surgery for biochemical analysis, while the remaining 10 mice from each group were monitored for survival over a 30-hour period. This design was intended to compare survival rates, with a focus on the 24-hour period following CLP surgery.
Exercise Training Protocol
Mice in the CLP-Ex group underwent a 5-day gradual acclimatization phase on the treadmill. During this phase, they ran for 20 minutes daily at speeds that varied from 8 to 15 meters per minute (m/min). The exercise regimen incorporated interval training, beginning with 2 minutes of running at 15 m/min, followed by 2 minutes of active recovery at 8 m/min. This pattern was repeated 10 times each day, continuing for a total of 8 weeks. The running speed was incrementally increased by 1 m/min each week, reaching a peak of 22 m/min during the final week of training.
CLP Surgery
Sepsis was induced using a modified version of the CLP method, as previously described [23]. Briefly, mice were anesthetized with intraperitoneal injections of Zoletil (50 mg/kg) and Rompun (10 mg/kg). The lower abdominal area was shaved, and a midline incision was made to access the peritoneal cavity. The linea alba, the midline white fascia of the abdominal musculature, was identified and carefully dissected to create an intermuscular incision. The cecum was then exposed and ligated approximately 1 cm from its tip using a 2-0 silk suture. A single puncture was made between the ligation and the cecal tip with a 25-gauge needle. After needle removal, the abdominal incision was closed with metallic clips. The mice were then returned to their cages in a temperature-controlled room maintained at 22┬░C.
Survival Curve
The survival of 10 mice in each group was closely monitored at regular 2-hour intervals for 30 hours following CLP surgery. The time of death for each mouse was noted. If a mouse died between the 2-hour observation points (for example, between 2 and 4 hours), its survival time was recorded as the higher value (for instance, 4 hours). If a mouse survived the entire 30-hour observation period, its survival time was recorded as 30 hours.
Western Blot
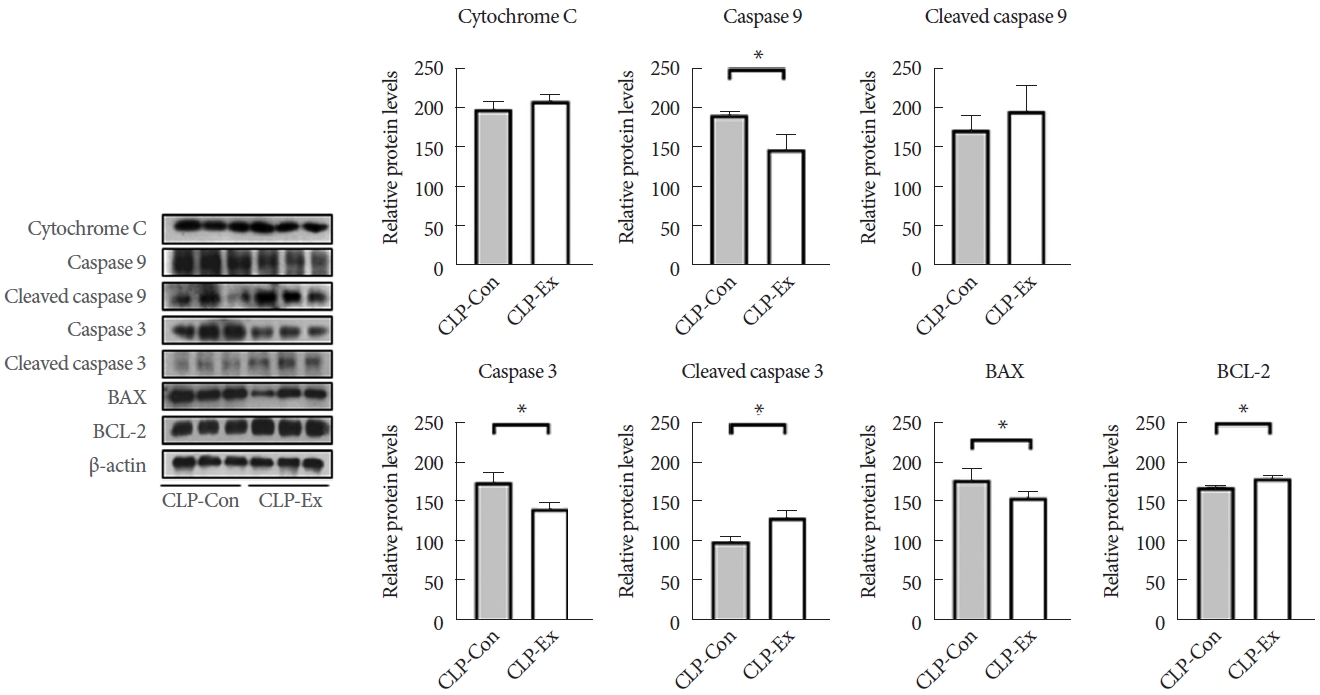
The hippocampus was homogenized and subsequently centrifuged to prepare protein extracts. After centrifugation, the supernatants were collected. Protein concentration was determined using the Bradford assay (Bio-Rad, Hercules, CA, USA). A total of 20 ╬╝g of protein was mixed with Laemmli sample buffer and boiled. For electrophoresis, these protein samples were loaded onto a sodium dodecyl sulfate/polyacrylamide gel with a gradient concentration ranging from 7.5% to 15%. After electrophoresis, the separated proteins were transferred onto a nitrocellulose membrane (Whatman, Dassel, Germany). The membranes were then blocked in a solution containing 5% nonfat dried milk and 0.05% Tween in Tris-buffered saline for 1 hour to prepare them for further analysis. Following the blocking step, the membranes were incubated with primary antibodies at 4┬░C overnight. The specific antibodies used were as follows: rabbit anti-TNF-╬▒ (1:1,000═Š ab6671═Š Merck Millipore, Darmstadt, Germany), rabbit anti-caspase 8 (1:1,000═Š #4790═Š Cell Signaling, Danvers, MA, USA), rabbit anti-cleaved caspase 8 (1:1,000═Š #8592═Š Cell Signaling), mouse anti-RIP1 (1:1,000═Š 610458═Š BD Biosciences, San Diego, USA), rabbit anti-phosphorylated (p)-RIP1 (1:1,000═Š #31122═Š Cell Signaling), rabbit anti-RIP3 (1:1,000═Š #95702═Š Cell Signaling), p-RIP 3 3 (1:1,000═Š #91702═Š Cell Signaling), rabbit anti-MLKL (1:1,000═Š #37705═Š Cell Signaling), rabbit anti-p-MLKL (1:1,000═Š #37333═Š Cell Signaling), rabbit anti-cytochrome C (1:1,000═Š #4272═Š Cell Signaling), rabbit anti-caspase 9 (1:1,000═Š ab184786═Š Abcam, Cambridge, UK), anti-p-caspase 9 (1:1,000═Š #9507═Š Cell Signaling), rabbit anti-caspase 3 (1:1,000═Š #9662═Š Cell Signaling), anti-pcaspase 3 (1:1,000═Š #9661═Š Cell Signaling), mouse anti-BAX (1: 1,000═Š sc-20067═Š Santa Cruz Biotechnology, Dallas, TX, USA), rabbit anti-BCL-2 (1:1,000═Š ab196495═Š Abcam), rabbit anti-4-hydroxynonenal (4-HNE) (1:1,000═Š ab46545═Š Abcam), rabbit anti-glutathione peroxidase (1:1,000═Š ab22604═Š Abcam), rabbit anti-SOD2 (1:1,000═Š sc-30080═Š Santa Cruz Biotechnology), rabbit anti-catalase (1:1,000═Š ab16731═Š Abcam), and rabbit anti-╬▓-actin (1:1,000═Š A300-491A═Š Bethyl, Montgomery, TX, USA). After incubation, blots were developed using a chemiluminescent horseradish peroxidase substrate kit (Millipore, Billerica, MA, USA) to visualize the protein bands. Band intensities were quantified using ImageJ version 1.42 (National Institutes of Health, Bethesda, MD, USA).
Immunohistochemistry
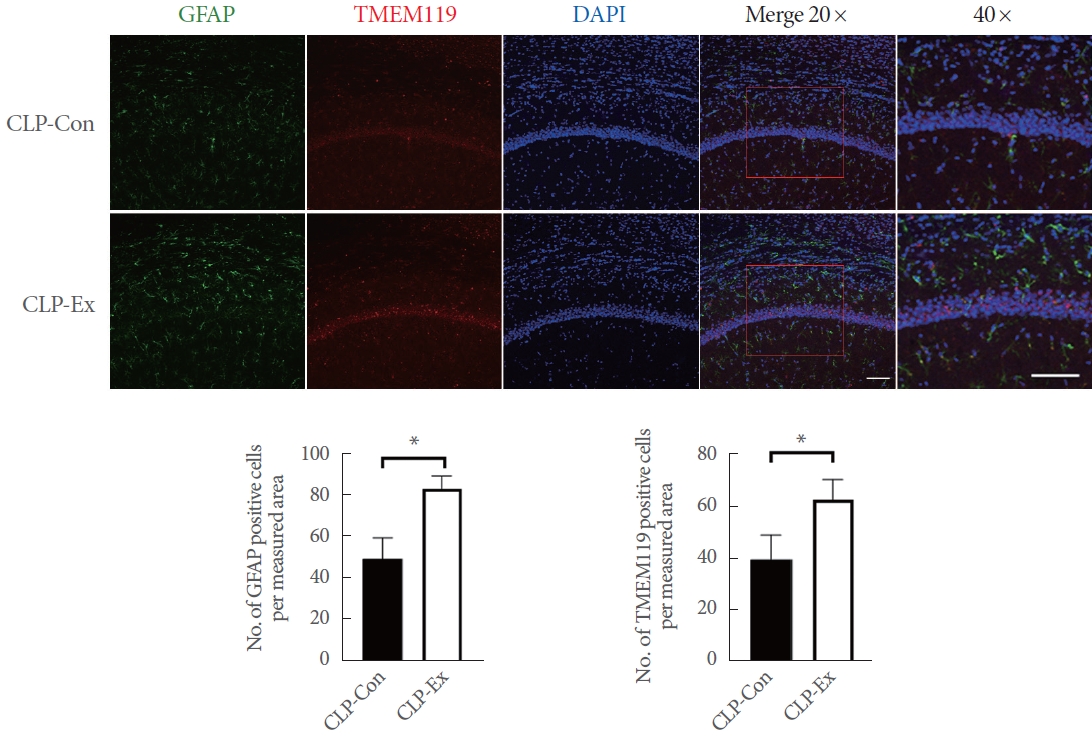
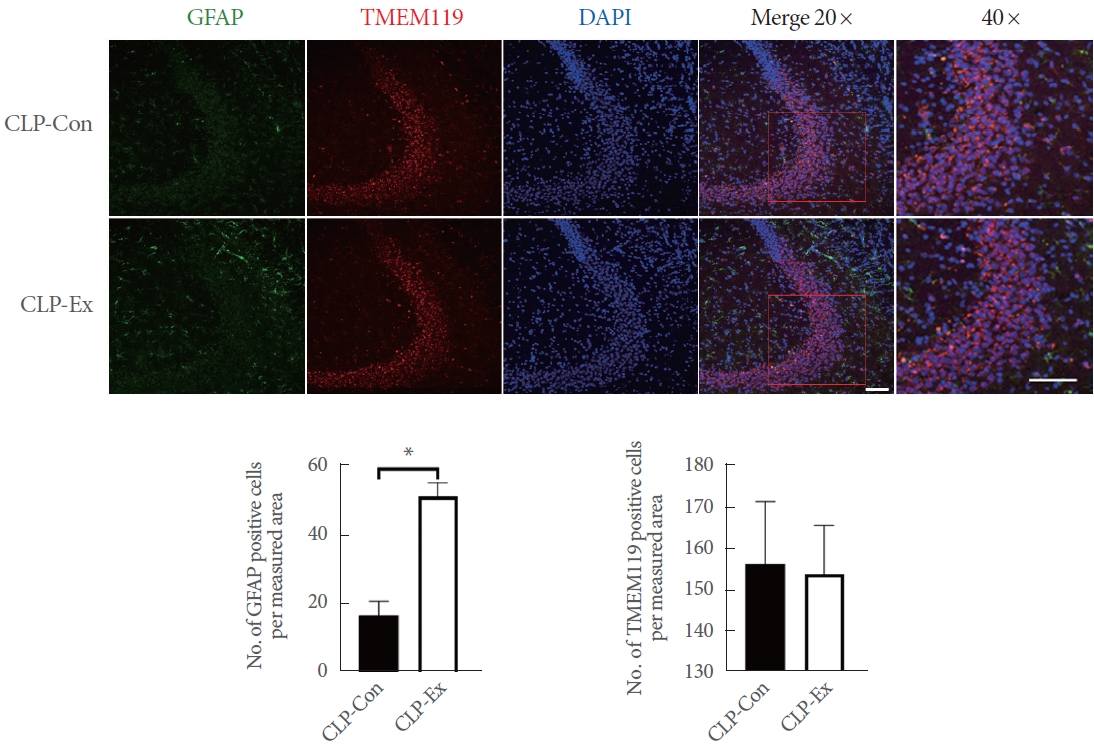
The brain was sectioned into 40-╬╝m-thick slices using a microtome (CM3050S; Leica Microsystems, Nussloch, Germany). These sections were then stored in a cryoprotection solution consisting of 20mM KH2PO4, 80mM KH2PO4, 0.3 g/mL sucrose, 154mM NaCl, 30% v/v ethylene glycol, and 0.01-g/mL polyvinylpyrrolidone. To prepare the brain sections for immunostaining, the slices were subjected to the following steps. First, they were pretreated with 70% formic acid for 10 minutes at room temperature. This was followed by 3 consecutive 5-minute washes in 1├Ś phosphate-buffered saline (PBS). Next, the sections were blocked with 5% goat serum for 30 minutes. The brain sections were then incubated with 2 primary antibodies: mouse antiglial fibrillary acidic protein (GFAP, MAB360; Millipore) and rabbit antimicroglia transmembrane protein 119 (TMEM119, ab209064; Abcam). After washing with PBS, the sections were incubated for 2 hours with secondary antibodies, specifically anti-mouse immunoglobulin G (IgG) Alexa Fluor-488 (A11034; Thermo Scientific, Rockford, IL, USA) and anti-rabbit IgG Alexa Fluor-594 (A11001; Thermo Scientific). The sections were stained with DAPI (Santa Cruz Biotechnology, Paso Robles, CA, USA) and mounted on glass slides for DNA examination. A specialist, who was blinded to the experimental protocol, examined the stained brain sections. Images were captured using a Zeiss LSM 510 META Duoscan confocal microscope (Zeiss, Oberkochen, Germany) equipped with a ├Ś20 objective. After image capture, cells were manually counted to determine the number of cells per measured area.
TUNEL Assay
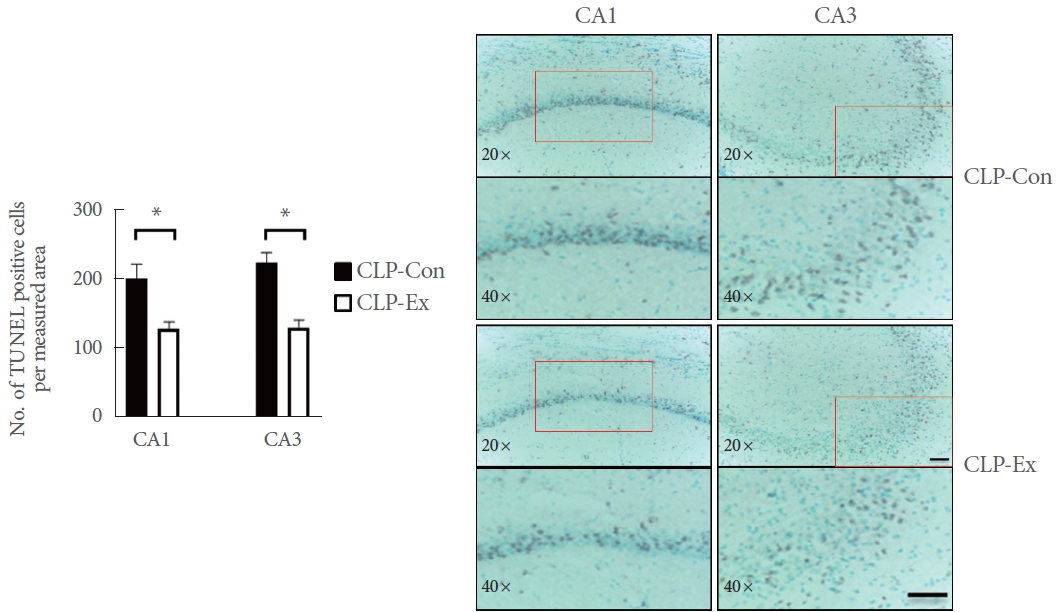
A terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay was performed on the brain tissue sections. First, the sections were permeabilized for 2 minutes with Triton X-100 at 4┬░C. Subsequently, they were immersed in TUNEL reagent for 60 minutes at 37┬░C. Morphological analysis of the stained slides was conducted using the Leica QWin Image Analyzer System, paired with a Leica DMLS microscope (Leica Microsystems). After image capture, cells were manually counted to determine the number of cells per measured area.
Statistical Analysis
The recorded values are presented as means with standard deviations. An independent t-test was used to assess potential statistically significant differences in measurements between the groups. Statistical analyses were conducted using IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA). P-values less than 0.05 were considered to indicate statistical significance.
RESULTS
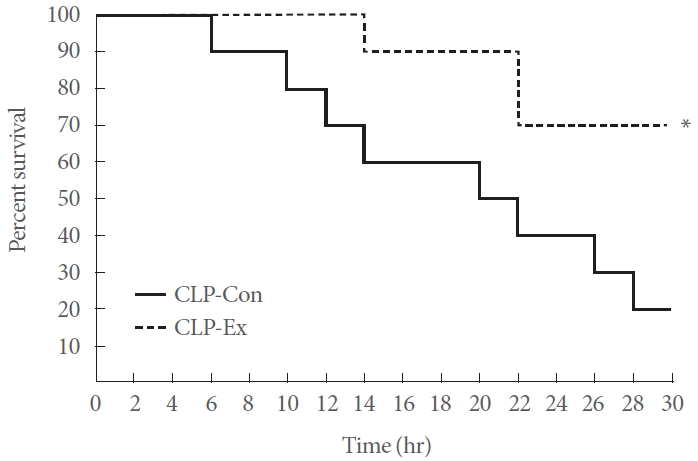
Exercise Training Increases Survival in CLP Mice
The Kaplan-Meier mortality curve revealed that the CLP-Ex mice exhibited a significantly lower mortality risk (P=0.043) than the CLP-Con mice after 30 hours of sepsis. The survival rate for the CLP-Ex mice was 70%, in contrast to a 20% survival rate for the CLP-Con mice (Fig. 2).
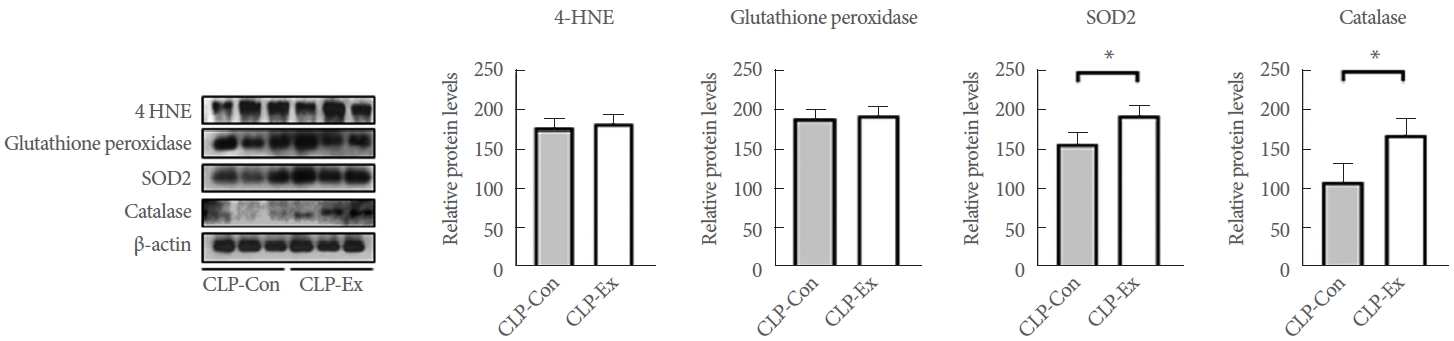
Exercise Training Enhances Antioxidative Capacity in the Hippocampus of CLP Mice
The levels of 4-HNE, glutathione peroxidase, SOD2, and catalase were measured to investigate the effects of exercise training on oxidative stress. As shown in Fig. 3, the expression level of 4-HNE was comparable between groups (P=0.591). Regarding antioxidative capacity, CLP-Ex mice exhibited significantly higher levels of SOD2 (P=0.026) and catalase (P=0.025) compared to CLPCon mice, although no significant difference was observed in glutathione peroxidase levels. These findings indicate that exercise training enhances the antioxidative capacity in the hippocampus of CLP mice.
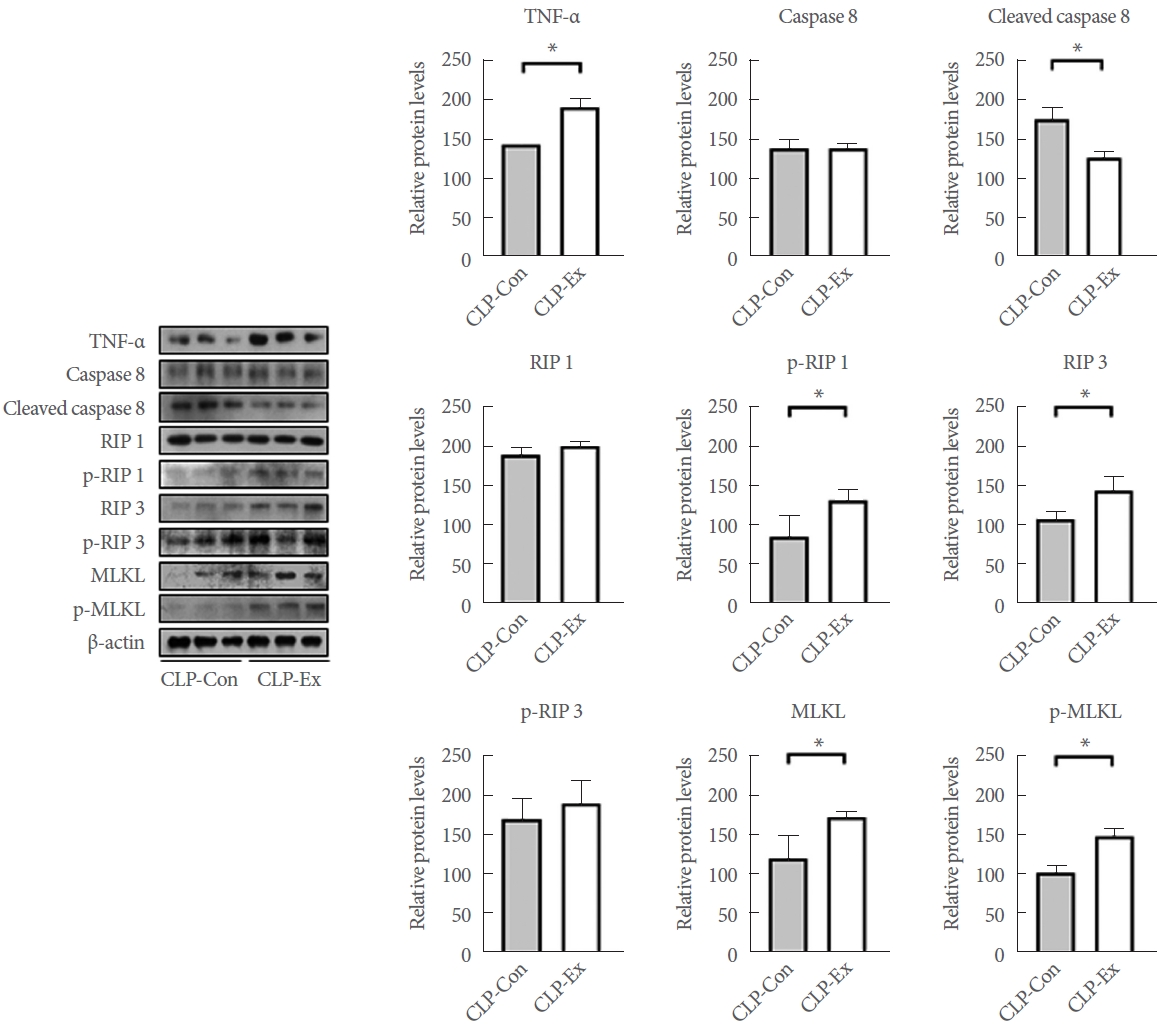
Exercise Training Delays the Transition From a Hyperdynamic to an Immunosuppressive State in CLP Mice
In terms of necroptotic cell death and proinflammation, the hippocampi of CLP-Con mice displayed significantly lower levels of TNF-╬▒ (P=0.046), cleaved caspase 8 (P=0.007), p-RIP1 (P=0.014), RIP3 (P=0.027), MLKL (P=0.042), and p-MLKL (P=0.003) expression compared to the CLP-Ex mice (Fig. 4). These findings align with the higher levels of inflammation markers GFAP and TMEM119 observed in the CA1 (P=0.008 and P=0.012, respectively) and CA3 (P=0.032 and P=0.821, respectively) regions of the hippocampus in CLP-Ex mice, relative to the CLP-Con animals (Figs. 5, 6). This suggests that the CLP-Ex mice experienced greater inflammation than the CLP-Con mice. In the context of apoptotic cell death, CLP-Con mice exhibited significantly higher levels of caspase 9 (P=0.016), caspase 3 (P=0.011), and BAX (P=0.043) expression, along with lower levels of BCL-2 (P=0.007), compared to CLP-Ex mice (Fig. 7). These findings align with the greater number of TUNEL-positive cells found in the CA1 and CA3 regions of the hippocampus in CLPCon mice (P=0.06 and P<0.01, respectively; Fig. 8). The data suggest that exercise training may delay the progression from a hyperdynamic state to an immunosuppressive apoptotic state in mice following CLP.
DISCUSSION
The present study offers meaningful insights into the effects of exercise training on survival rates in mice with CLP-induced sepsis. Our findings demonstrate that exercise training enhances survival outcomes, likely due to an extended transition from the hyperdynamic to the hypodynamic phase of sepsis. Notably, mice that underwent exercise training experienced a prolonged period of neural necroptosis and increased proinflammatory responses during this lengthened hyperdynamic phase compared to their untrained counterparts. A key observation from this study is the sustained elevation of TNF levels in trained mice, along with a continuous inflammatory response over the 24 hours following CLP. In contrast, untrained mice displayed lower TNF expression levels and a weaker inflammatory response during this period. These results imply that exercise training may foster a prolonged proinflammatory state, potentially enhancing the hostŌĆÖs defense mechanisms and leading to higher survival rates in mice with sepsis. Additionally, our study underscores the importance of exercise-induced antioxidant activity in mitigating the detrimental effects of sepsis-induced oxidative stress. We observed that exercise training increased antioxidant capacity, effectively counteracting the disruption caused by sepsis. This restoration of redox balance helps to alleviate the disparity between the increased production of ROS and antioxidant capacity, which is characteristic of sepsis. Consequently, exercise training is instrumental in reducing neural cell damage and delaying the onset of immunosuppression, thereby contributing to improved survival rates in septic mice.
The present findings align with prior research, indicating that exercise training has beneficial effects on survival in the context of sepsis. Deng et al. [22] observed a marked difference in the inflammatory state between trained and untrained mice 24 hours after CLP, a pivotal time when the body transitions from a proinflammatory to an immunosuppressive state. This suggests that exercise preconditioning may influence the inflammatory state transition in mice after CLP, contributing to an increased survival rate. Moreover, several studies have demonstrated that exercise intervention can reduce multiple organ damage and improve survival rates in various sepsis models. For example, Sossdorf et al. [24] reported that 6 weeks of treadmill and voluntary wheel running improved systemic immune responses and lessened kidney damage in a cecal slurry injection model. Similarly, De Ara├║jo et al. [20] showed that 8 weeks of regular, moderate treadmill running decreased lung and distal organ damage and improved survival in the CLP model by restoring the balance between inflammation and immune response. Additionally, Irahara et al. [21] recently reported that 3 days of low-intensity treadmill running during the hyperdynamic phase of sepsis lessened liver damage and improved survival by increasing fatty acid oxidation in LPS-treated mice. Collectively, these findings support the notion that exercise training confers protective effects against sepsis-induced organ damage and mortality, underscoring its potential as a therapeutic strategy in sepsis management. The consistency of our results with those of previous studies reinforces the robust and positive impact of exercise training on sepsis outcomes.
Our findings further revealed that exercise preconditioning increased the expression of antioxidants, which helped to counteract the heightened oxidative stress observed in CLP-Ex mice. This result is consistent with prior animal research indicating how exercise-induced redox balance can mitigate cellular damage during sepsis. For example, Cardoso et al. [25] observed that 3 weeks of high-intensity swimming in mice with sepsis increased the levels of antioxidant enzymes, including glutathione, SOD, and catalase. This was accompanied by reductions in inflammatory processes and lung damage. In a similar vein, Miron et al. [26] demonstrated that 12 weeks of climbing exercise in septic rats resulted in reduced lung damage and increased survival rates, which were attributed to enhanced antioxidant activity. Coelho et al. [27] corroborated these observations, demonstrating that 8 weeks of treadmill running improved antioxidant activity, effectively preventing lipid peroxidation and muscle cell damage in animals with sepsis.
The regulation of oxidative stress is critical for managing necroptosis and inflammatory responses. Previous studies have shown that using a ROS reducer, such as necrostatin-1, can reduce necroptosis in a variety of neurological conditions. Research conducted by Northington et al. [28], You et al. [29], and King et al. [30] has shown that necrostatin treatment decreases ROS accumulation, reduces necroptotic cell death, and lessens disease symptoms in conditions including neonatal hypoxiaischemia, traumatic brain injury, and intracerebral hemorrhage, respectively. Additionally, Rittirsch et al. [23] demonstrated the protective effects of necrostatin on cortical neurons in rats subjected to N-methyl-D-aspartateŌĆōinduced neurodegeneration. Therefore, it is reasonable to suggest that exercise training may protect the brain during the hyperdynamic phase of sepsis by attenuating excessive ROS production and necroptosis via the enhancement of antioxidative capability.
In addition to an increase in antioxidant levels, our study revealed that the delayed onset of immunosuppression in exercisetrained mice was associated with a decrease in apoptosis, which occurred due to exercise-induced apoptotic protein modulation. Animal studies conducted by Ko and Ko [31] and Song et al. [32] showed that both voluntary wheel running and treadmill exercise reduced LPS-induced neuroinflammation, leading to a lower BAX/BCL-2 ratio and diminished apoptosis. Aboutaleb et al. [33] provided further insights into the mechanisms underlying the antiapoptotic effects of exercise. In a model of cerebral ischemia, they found that 4 weeks of treadmill exercise modulated the BAX/BCL-2 ratio and reduced apoptosis by inhibiting the activation of caspase-3. These results indicate that exercise training might offer protective benefits against apoptosis in sepsis by altering the expression and activity of apoptotic proteins. Additionally, other proteins induced by exercise could play a role in the observed reduction in apoptosis. For instance, exercise has been found to boost the expression of Sirt-1, a protein that influences the function of poly (ADP-ribose) polymerase-1 and peroxisome proliferator-activated receptor gamma coactivator 1-alpha. These proteins are crucial in fat metabolism, the reduction of cellular load, and the regulation of ROS production and cell death [34,35]. Therefore, exercise training may attenuate apoptosis in sepsis through multiple pathways, including the modulation of apoptotic protein expression and activity.
Our study had several limitations. First, we focused exclusively on sepsis-associated encephalopathy. However, the survival rate of sepsis is closely tied to multiple organ dysfunction. Consequently, the effects of exercise on other organs merit additional research. Second, while prior studies such as the investigation by Deng et al. [22] have provided supporting data indicating immunosuppression, evidenced by reduced serum levels of both proinflammatory and anti-inflammatory cytokines, our study did not investigate a wider range of inflammation markers beyond Western blot data. This indicates that further evidence may be necessary to validate our findings. Third, our analysis was limited to quantifying the presence of microglia and astrocytes without examining the pro- and anti-inflammatory cells in detail, including their specific morphologies. Finally, our data were confined to the 24 hours following CLP surgery, emphasizing the further need to explore the long-term progression of inflammatory states after surgery.
In summary, our study highlights the diverse benefits of exercise training in the context of sepsis. Exercise training appears to be a promising therapeutic approach, as it promotes sustained proinflammatory responses and enhances antioxidant activity, potentially mitigating the detrimental effects of sepsis and improving survival outcomes. These findings underscore the importance of integrating exercise interventions into the clinical management of patients with sepsis, potentially improving their overall prognosis and outcomes. However, this study does not explore the dynamic changes in inflammatory states and cell death that occur after CLP, and our conclusions are limited by the scope of the data available. Future research should investigate the sequence of exercise-induced changes following CLP to elucidate the temporal impact of exercise training on sepsis.