Effect of Low-Dose Triple Therapy Using Gabapentin, Amitriptyline, and a Nonsteroidal Anti-Inflammatory Drug for Overactive Bladder Symptoms in Patients With Bladder Pain Syndrome
Article information
Abstract
Purpose:
Patients with bladder pain syndrome/interstitial cystitis (BPS/IC) can have pain as a main symptom and overactive bladder (OAB) symptoms that are directly or indirectly related to a major mechanism that causes pain. The primary purpose of this study is firstly to identify the prevalence rate of OAB symptoms in patients with BPS/IC, secondly to identify changes in OAB symptoms after low-dose triple therapy, and thirdly to build a theoretical foundation to improve quality of life for patients.
Methods:
Patients who met the inclusion criteria of BPS/IC through basic tests including the O’Leary-Sant symptom index, overactive bladder symptom score (OABSS), and visual analog scale (VAS) were identified. Treatment-based changes in OAB symptoms were identified using the IC Symptom Index and IC Problem Index (ICSI/ICPI), OABSS, and VAS before, and 4 and 12 weeks after low-dose triple therapy.
Results:
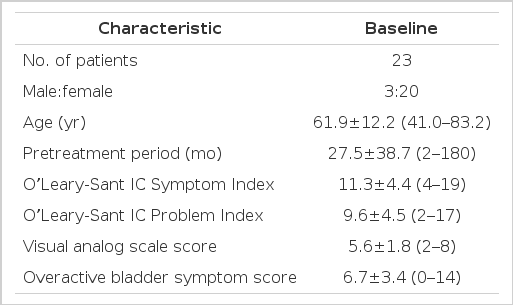
The patients consisted of 3 men and 20 women, and their mean age was 61.9 years (41.0–83.2 years). Comparing values before treatment, and 4 and 12 weeks after treatment (baseline vs. 4 weeks to baseline vs. 12 weeks), the rates of improvement were as follows: ICSI, 44.2% to 63.7%; ICPI, 46.9% to 59.4%; OABSS, 34.3% to 58.2%; and VAS, 53.6% to 75.0%, which showed statistically significant differences (P<0.05). However, comparing values at 4 and 12 weeks after treatment (4 weeks vs. 12 weeks), the ICSI and VAS showed a statistically significant decrease (P<0.05). The ICPI and OABSS showed slight improvement, but no statistically significant differences (P>0.05).
Conclusions:
Low-dose triple therapy in BPS/IC results in a clear decrease in OAB symptoms in the first 4 weeks after treatment, and additional treatment for 8 weeks had a partial effect with varied statistical significances depending on the questionnaires.
INTRODUCTION
Bladder pain syndrome/interstitial cystitis (BPS/IC) is defined as no infection or other clinically identified causes with lower urinary tract symptoms and unpleasant bladder-related sensations such as pain, pressure, and discomfort for ≥6 weeks [1,2]. Overactive bladder (OAB) is diagnosed on the basis of voiding symptoms such as frequency, urgency, nocturia, and sometimes urgency incontinence [3]. Patients with BPS/IC can have pain as a main symptom and OAB symptoms that are directly or indirectly related to a major mechanism that causes pain [2,3]. Thus, treatment of pain is the most important issue for patients with BPS/IC, and at the same time, proper treatment of the OAB symptoms that accompany pain or persist after pain treatment has a great influence on quality of life for patients.
While BPS/IC is characterized by pain, OAB may be characterized by incontinence or the fear of impending incontinence. As each of these primary symptoms has an influence on the perception of urgency and has slightly different characteristics, doctors might distinguish between the disorders if they analyze symptoms such as urgency through close observation. However, it is difficult to distinguish between two disorders by using symptoms such as urgency [1–4]. As diagnosis of BPS/IC and evaluation after treatment are based on symptoms, it is very important for examiners to clearly define and classify pain and OAB symptoms expressed by patients with BPS/IC.
The primary purpose of our study is firstly to identify the prevalence rate of OAB symptoms in patients with BPS/IC, secondly to identify changes in OAB symptoms after low-dose triple therapy by using gabapentin, amitriptyline, and a nonsteroidal anti-inflammatory drug (NSAID), and thirdly to build a theoretical foundation to improve quality of life for patients.
MATERIALS AND METHODS
This is a prospective, open-label study of 23 patients who were diagnosed with BPS/IC at Wonkwang Urologic Clinic between September 2011 and December 2012. Study procedure was approved by the Institutional Review Board at Wonkwang University School of Medicine & Hospital. Patients who met the inclusion criteria of BPS/IC through basic tests including the O’Leary-Sant Symptom Index (OLS), overactive bladder symptom score (OABSS), and visual analog scale (VAS) were included in the study. The OLS was divided into the IC Symptom Index (ICSI) and the IC Problem Index (ICPI) for measurement [2,3,5]. Twenty-three patients completed questionnaires before treatment and 4 weeks after treatment, and 13 patients completed questionnaires 12 weeks after treatment.
All patients diagnosed with BPS/IC were primarily treated with low-dose triple therapy using an NSAID, amitriptyline, and gabapentin for 12 weeks. They were instructed to take 600 mg of micronized etodolac, 5 mg of amitriptyline, and 300 mg of gabapentin before sleeping. Previous or current intake of NSAIDs, amitriptyline, gabapentin, and anticholinergic agents was considered an exclusion criterion for study enrollment.
To evaluate the validity of this study, changes in OAB symptoms such as urgency, urgency incontinence, frequency, and nocturia for patients with BPS/IC after low-dose triple therapy were compared and identified. Treatment-based changes in OAB symptoms were identified using the ICSI/ICPI, OABSS, and VAS before treatment, and 4 and 12 weeks after low-dose triple therapy.
We applied methods such as the Wilcoxon signed rank test and Kruskal-Wallis test using SPSS ver. 13.0 (SPSS Inc., Chicago, IL, USA) as a statistical analysis. A P-value of ≤0.05 was considered statistically significant.
RESULTS
The patients consisted of 3 men and 20 women, and their mean age was 61.9 years (41.0 to 83.2 years) (Table 1). All patients experienced OAB symptoms such as urgency, nocturia, frequency, and urgency incontinence along with lower abdominal pain for ≥6 weeks, with a mean symptom period of 27.5 months before they visited the urologic clinic at Wonkwang University School of Medicine & Hospital. The ICSI, ICPI, OABSS and VAS tests conducted before treatment showed high scores of 11.3, 9.6, 6.7, and 5.6, respectively (Table 1). After 4 weeks of treatment, the ICSI, ICPI, OABSS, and VAS improved to 6.3, 5.1, 4.4, and 2.6, respectively (P<0.05). After 12 weeks of treatment, the ICSI, ICPI, OABSS, and VAS decreased to 4.1, 3.9, 2.8, and 1.4, respectively (P<0.05) (Table 2, Fig. 1). Comparing values before treatment, and 4 and 12 weeks after treatment (baseline vs. 4 weeks to baseline vs. 12 weeks), the rates of improvement were as follows: ICSI, 44.2% to 63.7%; ICPI, 46.9% to 59.4%; OABSS, 34.3% to 58.2%; and VAS, 53.6% to 75.0% which showed statistically significant differences (P <0.05). However, comparing values at 4 and 12 weeks after treatment (4 weeks vs. 12 weeks), the ICSI and VAS showed a statistically significant decrease (P<0.05). The ICPI and OABSS showed slight improvement, but no statistically significant difference (P>0.05) (Table 2, Fig. 1).
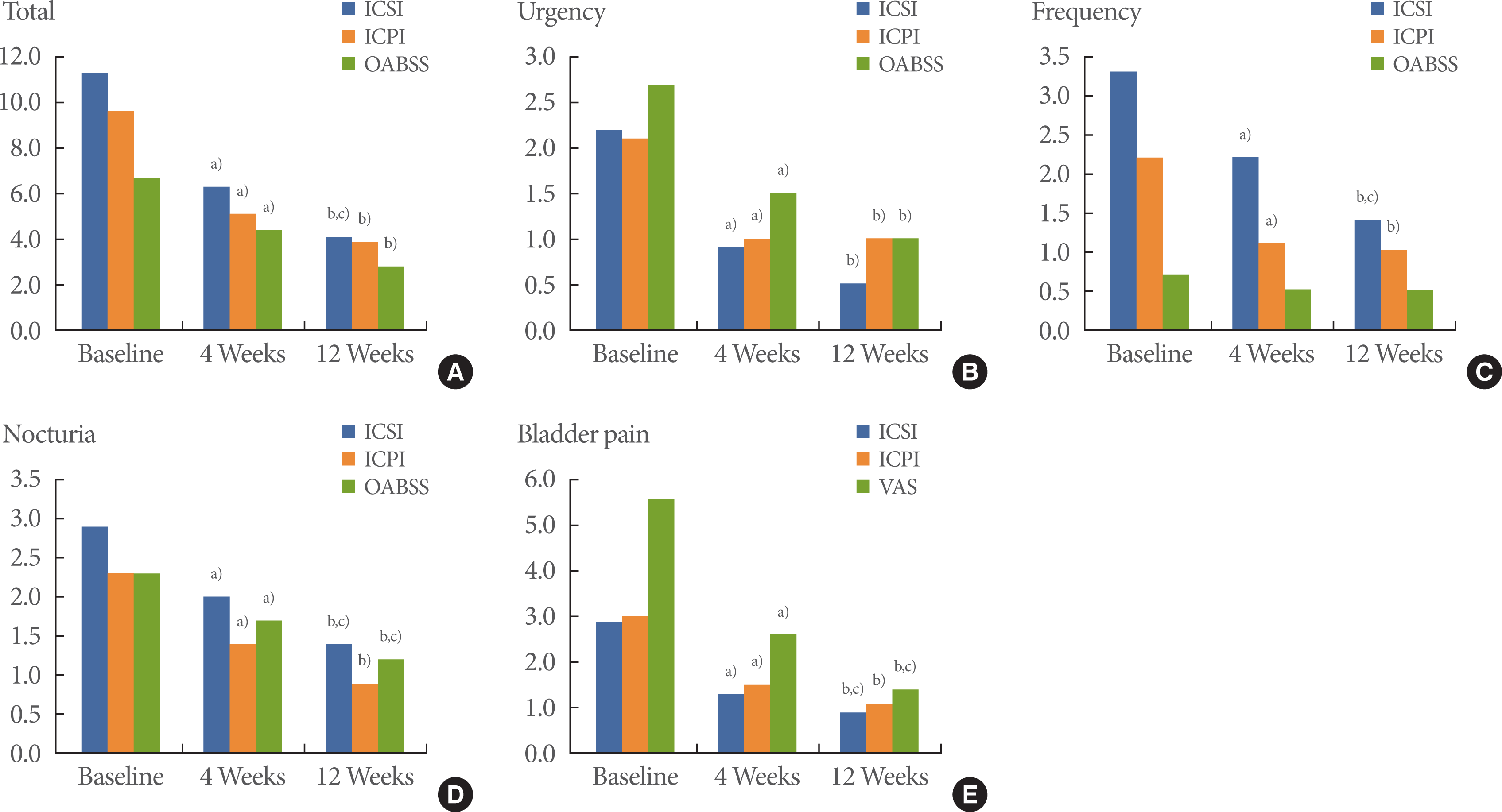
Changes in overactive bladder symptoms from baseline to 4 and 12 weeks. ICSI, Interstitial Cystitis Symptom Index; ICPI, Interstitial Cystitis Problem Index; OABSS, Overactive Bladder Symptom Score; VAS, visual analog scale. a–c)P<0.05 (a)baseline vs. 4 weeks; b)baseline vs. 12 weeks; c)4 weeks vs. 12 weeks) by Wilcoxon signed rank test.
Scores of urgency, urgency incontinence, frequency, nocturia, and bladder pain, which are components of the ICSI, ICPI, and OABSS, were compared before treatment and after treatment. As for the ICSI, a comparison of the results before treatment and 4 and 12 weeks after treatment revealed a statistically significant improvement in urgency, urgency incontinence, frequency, nocturia, and bladder pain (P<0.05). When results at 4 and 12 weeks after treatment were compared, urgency incontinence, frequency, nocturia, and bladder pain, but not urgency, showed a statistically significant improvement. A comparison of the ICPI results before treatment and 4 and 12 weeks after treatment indicated a statistically significant improvement in urgency (except baseline vs. 12 weeks), urgency incontinence, frequency, nocturia, and bladder pain (P<0.05). However, results at 4 and 12 weeks after treatment were compared, and both showed slight improvement but no statistically significant difference (P>0.05). Comparing the OABSS results before treatment and 4 and 12 weeks after treatment indicated a statistically significant improvement in urgency and nocturia alone (P<0.05). However, when results at 4 and 12 weeks after treatment were compared, nocturia showed a statistically significant improvement, while the other symptoms did not (P>0.05) (Table 2, Fig. 1).
Evaluation of the differences between the two questionnaires revealed that urgency, urgency incontinence, frequency, and nocturia according to the ICSI/ICPI and OABSS did not statistically differ; therefore, we confirmed that the symptoms were not underestimated by the ICSI/ICPI as compared to the OABSS.
Medication-related complications included drowsiness (n=5) and dry mouth (n=8); however, they were not serious enough to stop the treatment.
DISCUSSION
BPS/IC shows diverse variations depending on nations, races, and definitions. The occurrence rate of BPS/IC is estimated to be approximately 0.3%, of which 10% to 20% is in men. However, a population-based study by using questionnaires may show 30 to 50 times more frequent. Compared to the prevalence rate of OAB, that of BPS/IC is estimated to be ≤1/10 on average [1,2].
BPS/IC is defined as no infection or other identified causes with lower urinary tract symptoms and unpleasant bladder-related sensations such as pain, pressure, and discomfort for ≥6 weeks. OAB is diagnosed on the basis of voiding symptoms such as frequency, urgency, nocturia, and sometimes urgency incontinence [1–3].
Patients with BPS/IC can have pain as a major symptom and OAB symptoms directly and indirectly related to a major mechanism that causes pain [2,3]. Thus, treatment of pain is the most important issue for patients with BPS/IC, and at the same time, proper treatment of the OAB symptoms that accompany pain or persist after pain treatment has a great influence on quality of life for patients. It is estimated that the BPS/IC symptoms basically occur because of pain [6–10]. Thus, we primarily place the therapeutic focus on pain rather than the secondary symptom such as OAB. We first administered low-dose triple therapy by using gabapentin, amitriptyline, and an NSAID that caused a reduction in pain for months. Then, we measured the remaining OAB symptoms after treatment.
While urgency is related to relief of pain in BPS/IC, it may occur because of incontinence or the fear of impending incontinence in OAB. As for temporal characteristics of urgency, BPS/IC mainly involves persistent or sudden urge because of pain (persistent and/or sudden), and OAB mainly involves sudden urgency because of incontinence or the fear of impending incontinence (sudden). As the symptom profiles of the 2 diseases overlap, they appear similar. The major causes of urgency influence the perception of urgency, and they have slightly different characteristics. However, it is difficult to distinguish between the 2 diseases using urgency [11,12].
The International Continence Society defines urgency as “a sudden compelling desire to pass urine which is difficult to defer” [13]. The definition of urgency in the ICSI/ICPI widely used in questionnaires of BPS/IC is a “strong need to urinate with little or no warning” [14]. Urgency for most patients with BPS/IC is “difficult to postpone” (sudden), and at the same time, patients often note, “I always have warning.” That is, with a certain level of warning or more, suddenly “a severe compelling urge to urinate” (sudden) occurs. Therefore, “with little or no warning” in the ICSI/ICPI may underestimate the degree and prevalence of urgency [15,16]. However, when urgency is compared between the ICSI/ICPI and the OABSS in our findings, there is no statistical difference. This confirms that urgency in the ICSI/ICPI is not underestimated in our findings.
Greenberg et al. [15] explored the perceived causes of urgency; in 65% of patients with BPS/IC, it was associated with a desire to relieve pain, and in 21%, it was associated with a fear of incontinence. Only 6% of patients reported fear of incontinence without a linkage to pain. A total of 19% of patients with BPS/ IC did not report any urgency, although they did have frequency and nocturia. In order to explain urgency in patients with BPS/IC, it is appropriate to measure both persistent urge and sudden urgency precisely by using “desperate desire to void because of pain and fear of worsening pain” or “a compelling urge to urinate which is difficult to postpone” [16].
However, urgency questions used in the ICSI/ICPI and OABSS questionnaires to measure OAB symptoms all include sudden urgency only. There must be a limitation in precisely expressing urgency in patients with BPS/IC who have a persistent urge because of pain. Therefore, it is necessary to develop a new questionnaire that includes all sudden or persistent urgency based on fear of incontinence, relief of pain, or other motivations in the future.
Several causes of frequency and nocturia in patients with BPS/IC may be estimated. First, urgency because of pain can be a major cause. Second, it may occur because normal functional bladder capacity cannot be maintained because of pain itself or in order to decrease pain. Third, it may occur because urine in-flux into the bladder caused by bladder epithelial defect causes fibrosis of the bladder and low bladder capacity in the long term [12,13,17]. However, patients with BPS/IC often limit fluid intake consciously or subconsciously, and as a result, they may not suffer from frequency and nocturia.
In 6 patients with BPS/IC and significant remaining OAB symptoms after low-dose triple therapy, we used antimuscarinic agents and identified effects and side effects. After low-dose triple therapy for 4 weeks, we selected cases that showed overall points of ≥3 in OABSS and ≥2 in urgency. We added an anti-cholinergic, solifenacin 5 mg (Vesicare, Astellas Pharm Manufacturing Inc., Tokyo, Japan), to low-dose triple therapy and investigated the findings after administration for 8 weeks. When treatment before and after anticholinergic administration was compared, scores of urgency, urgency incontinence, frequency, nocturia, and bladder pain, which are components of the ICSI, ICPI, and OABSS, showed slight improvement but no statistically significant differences (P>0.05). It is, however, uncertain that this improvement was caused by pain control or the added anticholinergics (unpublished data).
According to our findings, when values before treatment and 4 and 12 weeks after treatment were compared, those of the ICSI, ICPI, OABSS, and VAS showed statistically significant improvement (P<0.05). However, when values 4 and 12 weeks after low-dose triple therapy were compared, the ICSI and VAS (P<0.05) showed contrasting results with the ICPI and OABSS (P>0.05).
In conclusion, low-dose triple therapy in BPS/IC showed a clear decrease in OAB symptoms for the first 4 weeks after treatment, and additional treatment for 8 weeks had a partial effect with varied statistical significances depending on the questionnaires.
Acknowledgements
This work was supported by Wonkwang University in 2013.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.