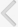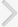INTRODUCTION
Acetylcholine (Ach) is one of the major regulators of urinary bladder function and is mainly released from the postganglionic fiber terminals to induce contraction [1]. However, a nonneuronal release of Ach has been shown in the bladder, bronchial, and corneal epithelia and digestive tract mucosa. These nonneurogenic Ach molecules act in an autocrine or paracrine manner [2,3]. Similarly, urothelium also secretes neurotransmitters in response to extrinsic stimulation when the bladder is filled and distended; Ach is released particularly in the bladder filling phase [4,5]. According to several studies, the urothelium acts primarily as a barrier to urine but also expresses various types of receptors and responds actively to extrinsic stimulation, thus helping to regulate bladder function [6,7].
Several studies have been conducted to examine the muscarinic and purinergic receptors in the urothelial layer of the bladder. It has been postulated that the nicotinic Ach receptor (nAChR) might be associated with the induction of overactive bladder (OAB) symptoms [8,9]. nAChR regulates bladder function by mediating fast synaptic transmission and is also involved in the contraction and relaxation of the bladder in the autonomic nervous system [5]. To date, 17 types of nAChR have been reported, including 10 alpha (α) subtypes and 4 beta (β) subtypes. In addition, there are gamma (γ), delta (δ), and epsilon (ε) subtypes [10-14]. Beckel et al. [15] reported that the α3 and α7 type nAChRs in the urothelium of rats were involved in regulating bladder function, and the inhibition of α3 receptors resulted in an inhibition of reflex voiding, but the inhibition of α7 receptors reduced the inhibition of reflex voiding.
Bladder outlet obstruction (BOO) is one of the causes for OAB. OAB arises from the functional, pharmacological, and molecular biological changes following the onset of BOO. Of these, changes in various types of receptors present in the bladder have been of increasing interest, and to date, studies have focused on muscarinic receptors [16]. However, little is known about the role of nAChRs.
Therefore, we attempted to examine the expression of α3 and α7 nAChRs in the bladder. We used rats with detrusor overactivity induced by partial BOO. We also investigated the changes in the functions of the bladder corresponding to the subunits of nAChR following the administration of specific antagonists of the α3 and α7 nAChRs.
MATERIALS AND METHODS
Experimental Animals
We used 40 male Sprague-Dawley rats, weighing 450 to 500 g (16 weeks old). They were divided into control group (n=10) and BOO-induced group (n=30). For the control group, a sham operation was performed. In the BOO-induced group, a partial BOO was attempted. Animals were handled in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and experimental protocols were approved by the Institutional Animal Care (CUMC-2008-0012-02) and Use Committee of the Catholic University of Korea.
Partial BOO
Experimental animals were anesthetized using an intramuscular injection of 15-mg/kg ketamine (Yuhan Co., Seoul, Korea) and 5-mg/kg xylazine (Rompun, Bayer Korea Co., Seoul, Korea). With the rats in the supine position, a longitudinal incision was made in the lower abdomen. The bladder neck and periurethral area were dissected out. A 25-G needle sheath was inserted into the urethra and the bladder neck was ligated using a 3-0 silk, after which the sheath was removed. In the sham-operated control animals, the bladder neck was very loosely ligated to not induce any obstruction.
Assessment of Bladder Functions
Three weeks following the obstruction, cystometrogram was performed in the control and BOO-induced groups. Of the 30 rats with BOO, 10 were given an intravesicular administration of the α3 nAChR antagonist, hexamethonium (HM group); 10 were administered the α7 nAChR antagonist, methyllycaconitine citrate (MLC group), and 10 were received only saline infusion (BOO group).
A suprapubic midline incision was made under urethane anesthesia (12 mg/kg) and the bladder was identified. A 25-G needle connected to polyethylene tubing was placed in the bladder dome. The tubing was connected to a pressure transducer and an infusion pump using a 3-way stopcock. The bladder was emptied and saline was infused into the bladder at 0.04 mL/min using a Harvard syringe pump. Intravesical pressure was recorded using a polygraph apparatus (Grass 7D, Grass Instrument Co., Quincy, MA, USA); the contraction interval, contraction pressure, and presence of nonvoiding contraction (NVC) were examined. NVCs were defined as the rise in intravesical pressure exceeding 4 cmH2O during the filling phase but not during voiding of urine. The Harvard syringe pump was stopped in the HM and MLC rats and the bladder was emptied for the administration of the nAChR antagonists. Voiding activity was prevented by clamping the penis. Saline infusions (0.2 mL) containing 80mM HM or 1mM MLC were instilled using a 25-G needle placed superior to the bladder. One hour later, saline was infused into the bladder at 0.04 mL/min using a Harvard syringe pump. The BOO group also received the same treatment without the drugs for 1-hour bladder retention and the intravesicular pressure was recorded.
Tissue Extraction
After the bladder function test, the bladder and urethra of the control and BOO-induced groups were dissected from the adjacent tissue. The bladder was cut above the trigone region and was used for immunofluorescent staining and Western blot analysis.
Immunofluorescent Staining of α3 and α7 nAChRs
Thawed tissue samples (stored in liquid nitrogen) were fixed in 4% paraformaldehyde and placed in 0.5M sucrose at 4°C. Using the optimal cutting temperature solution, polyethylene glycol, the tissues were sectioned at 3 μm. The tissue sections were then rinsed with phosphate buffered saline (PBS; pH 7.4). To inhibit nonspecific immunofluorescence staining, slides were exposed to a blocking solution (1.5% normal goat serum, 1.5% normal horse serum, 1% BSA, 0.1% Triton X-100 in PBS) at room temperature for 1 hour. Antibodies to α3 nAChR (sc- 1771; 1:200; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) and α7 nAChR (sc-5544; 1:200; Santa Cruz Biotechnology Inc.) were then applied and incubated for 2 hours at room temperature. The slide was rinsed with PBS three times and then incubated in the secondary antibody, Alexa Fluor 488 labeled goat antirabbit IgG (1:300; Molecular Probes, Eugene, OR, USA) at room temperature for 1 hour. The sample was rinsed with PBS three times. Slides were stained with 4’,6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA, USA), and mounted for examination using a light microscope (Axiovert 200, Zeiss, Oberkochen, Germany). The immunoreactivity was expressed as mean±standard error of the mean (SEM) using a scoring system according to the degree of staining (5, very strong; 4, strong; 3, intermediate; 2, weak; 1, very weak). Thus, the distribution and intensity of α3 and α7 nAChRs were measured by 1 urologist and 2 pathologists.
Western Blotting
The tissue was ground in liquid nitrogen and processed using a Qproteome Mammalian Protein Prep Kit (Qiagen, Hilden, Germany). The prepared sample was centrifuged at 4°C and 14,000 rpm for 10 minutes. Total protein was measured using a Bradford dye-binding protein assay kit (Bio-Rad Laboratories Inc., Hertfordshire, CA, USA) according to the manufacturer’s instructions.
Protein (30 μg) and 2× sample buffer (Bio-Rad Laboratories Inc.) were mixed at a ratio of 1:1. The reaction was performed at 100°C for 5 minutes and then stopped at 4°C. Electrophoresis was performed at 4°C and 70 V for 2 hours, using a Mini-PROTEAN three-cell unit (Bio-Rad Laboratories Inc.). Isolated proteins were transferred to a polyvinylidene difluoride membrane (Millipore Corporation, Bedford, MA, USA) using a transblot SD semidry system (Bio-Rad Laboratories Inc.) based on six gels at 4°C at a voltage of 20 V for 90 minutes. The membrane was blocked using 10% skim milk. Primary antibodies to α3 nAChR (57 KDa: ab65181, Abcam PLC, Cambridge, UK), α7 nAChR (56 KDa: ab24644, Abcam PLC), and glyceraldehyde 3-phosphate dehydrogenase (36 KDa: ab37168, Abcam PLC) were diluted 1:500 using 5% skim milk. The reaction was run at room temperature for 1 hour. After the membrane was rinsed, enzyme-conjugated secondary antibodies, diluted in 5% skim milk at a ratio of 1:10,000, to α3 nAChR and glyceraldehyde 3-phosphate dehydrogenase were reacted with rabbit IgG-HRP (ab6721: Abcam PLC) and that of α7 nAChR was reacted with rat IgG-HRP (ab6734: Abcam PLC) at room temperature for 30 minutes. After the membrane was rinsed, the reaction using enhanced chemiluminescence solution (Amersham Biosciences, Buckinghamshire, UK) was performed for 1 minute. The membrane was placed on a photographic film and developed. Using a densitometer (LAS-3000, Fuji Film, Tokyo, Japan), quantitative analyses of α3 and α7 nAChRs levels were performed for the urothelium and detrusor muscle in the control and BOO groups.
Statistical Analysis
All data were analyzed using SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA). The data were expressed as mean±SEM. For comparisons among the groups, one-way analysis of variance and Newman-Keuls multiple comparison test were performed with P<0.05 as an indication of statistical significance.
RESULTS
Changes in the Contraction Interval and Contraction Pressure of the Bladder in Cystometrogram
In cystometrogram, the contraction interval of the BOO group (4.13±0.15 minutes) decreased significantly compared with the control group (10.25±0.17 minutes) (P<0.05). NVCs were observed in the BOO group. In the HM group, the contraction interval was 4.65±0.24 minutes, and there was no significant difference compared with the BOO group. However, the contraction interval of 7.55±1.12 minutes in the MLC group was significantly longer than that in the BOO group (P<0.05) (Fig. 1A). In the HM and MLC groups, NVCs were almost lost compared with the BOO group.
The contraction pressure of the BOO group was 30.12±1.38 cmH2O and significantly higher compared with the control group (20.95±0.92 cmH2O) (P<0.05). The contraction pressures of the HM and MLC groups were 19.08±1.53 and 13.53±1.02 cmH2O, respectively, and significantly lower compared with the BOO group (P<0.05) (Fig. 1B).
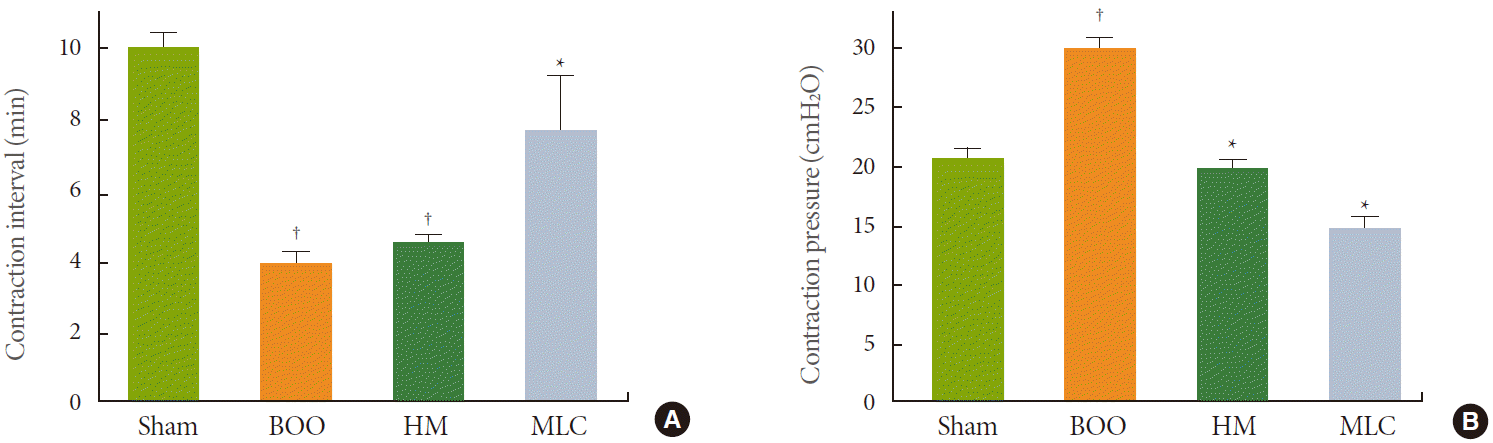
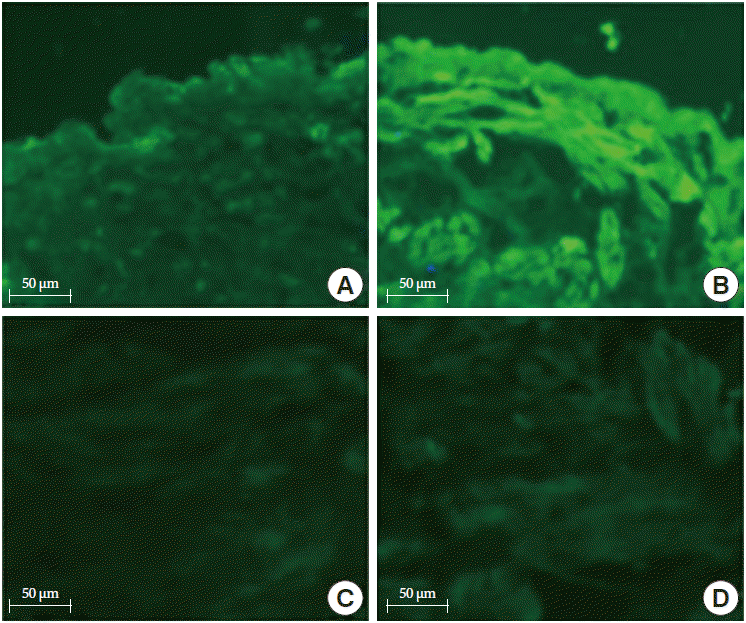
Changes in the Distribution and Expression of α3 and α7 nAChRs by Immunofluorescence
The α3 nAChR expression was observed mainly in the urothelium but had weak expression in the detrusor muscle layer. In the control group, it was observed mainly in the luminal surface of the urothelium. In the BOO group, the expression of α3 nAChR was significantly increased in the urothelium compared with the control group. However, there was no significant difference from the control group in the detrusor muscle layer (Fig. 2). The α7 nAChR expression was observed in both the urothelium and detrusor muscle, unlike α3 nAChR. In the control group, α7 nAChR expression was observed in all layers of the urothelium. In the BOO group, α7 nAChR expression further increased in the urothelium and detrusor muscle compared with the control group (Fig. 3).
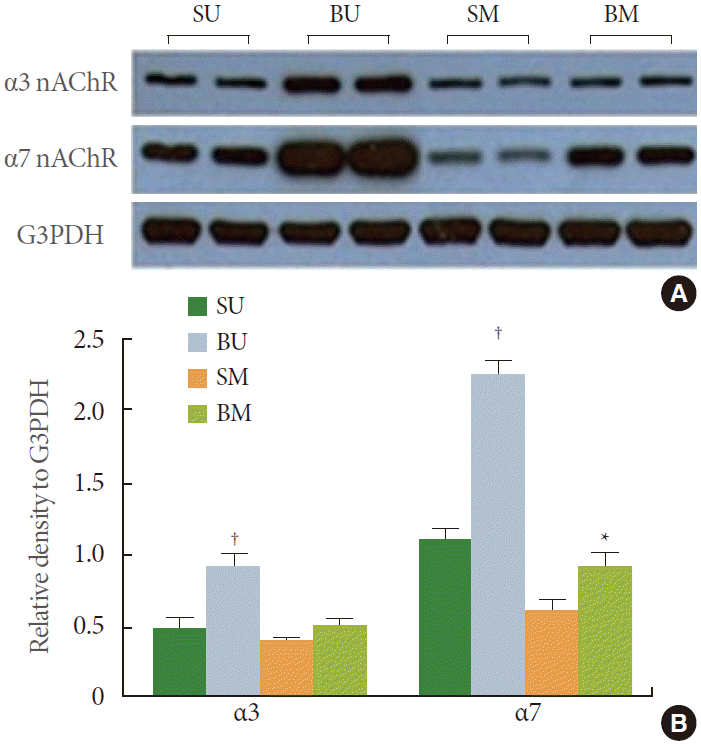
Changes in the Expression of α3 and α7 nAChRs by Western Blot Analysis
Expressions of α3 nAChR and α7 nAChR were confirmed in the mucosa and detrusor muscle. Alpha3 nAChR expression was significantly increased in the mucosa of the BOO group compared with the control group (P<0.05). However, the expression levels in the detrusor muscle were similar for the BOO and control group. Expression of α7 nAChR in the BOO group significantly increased both in the mucosa and detrusor muscle compared with the control group (P<0.05) (Fig. 4). These results were in accordance with the immunofluorescence result.
DISCUSSION
In our study, the distributions of α3 and α7 nAChRs were examined in rats in which detrusor overactivity was induced by partial BOO. Although this animal model does not represent detrusor overactivity in humans completely, it has been a mainstay of methods to generate OAB animal models according to several reports [17,18].
The α3 nAChR in the urothelium might play a role in detrusor hyperactivity caused by BOO. So far, little is definitively known about the expression and distribution of α3 nAChR in the OAB. Several studies has reported that α3 nAChR is involved in bladder contraction. De Biasi et al. [1] reported that mutant mice lacking the α3 nicotinic subunits developed bladder overdistention, and bladder strips from α3 null mice did not respond to nicotine. Vural et al. [19] reported that HM, a known α3 nAChR antagonist, inhibited the nicotine-induced increase in the electrical field stimulation-evoked contraction in the rabbit bladder. It can be inferred that the increased levels of α3 nAChR and changes in its distribution as well as the structural changes of urothelium caused by BOO might be key factors causing functional changes in the bladder. In addition, definitive information about the expression and distribution of α7 nAChR in the OAB is lacking. Bschleipfer et al. [20] reported that of the subunits of nAChR, α7 was more expressed and distributed in the human urothelium without detrusor hyperactivity. Alpha7 nAChR is more likely involved in the regulation of detrusor function. This was confirmed by the findings that the contraction interval was significantly increased, contractile force was decreased, and NVC of the bladder was lost in the cystometrogram in the MLC group compared with the BOO group in our study.
Both immunofluorescence and Western blot analysis confirmed the presence of nAChRs in the urothelium and detrusor muscle. The α3 nAChR in our study was expressed in the luminal surface of the bladder in the control group. In the BOO group, the α3 nAChR level increased in the urothelium. Alpha7 nAChR expression was observed in all layers of the urothelium. The expression of α7 nAChR in the BOO group significantly increased compared with the control group. Immunofluorescence and Western blot analysis showed that α7 nAChR expression was increased compared with α3 nAChR in the bladder with detrusor hyperactivity.
Changes in detrusor overactivity were monitored following the intravesicular administration of antagonists against α3 and α7 nAChRs. There was no significant change in the contraction intervals in the HM group compared with the BOO group. However, in the HM group, NVC of the bladder was almost lost. The contraction pressure was also significantly decreased compared with the BOO group. These results might relate to the α3 nAChR inhibitor, HM and are in agreement with other reports that afferent nerve conduction is activated when α3 nAChR releases adenosine triphosphate, an excitatory mediator, in response to extrinsic stimulation or extension of the urothelium [15,21,22]. The cause of a decrease in the contraction pressure in the HM group may be because the BOO damaged the urothelium, which increased the permeability of the urothelium to the HM. Hence, HM might have affected the urothelium and detrusor muscle, or the nerve below the urothelium directly. In general, HM mostly affects the α3 nAChR present in the urothelium of the normal bladder and has no significant effect on the detrusor muscle. However, in the obstructioninduced detrusor, HM has a direct effect on the detrusor muscle, nerve function of the bladder, as well as the urothelium. Therefore, contraction of the bladder could be suppressed.
In cystometrogram, the contraction interval significantly increased in the MLC group compared with the BOO group. NVC were almost absent and the contraction pressure decreased. According to the other studies, stimulation of α7 nAChR induces the secretion of nitric oxide, an inhibitor of normal urinary bladder function, and thereby, interferes with the neurotransmission of afferent excitation [23-27]. However, those studies differed from our results. Similar to the HM group, MLC may have a direct effect on α7 nAChR in the detrusor muscle as well as the urothelium due to the increased permeability of the urothelium caused by the structural changes and damage by BOO. This might lead to the suppression of detrusor hyperreflex. This postulation was in agreement with the report by Beckel et al. [15] that the urothelium is damaged following the intravesicular administration of protamine sulfate in rats with normal bladder function. They also noted the functional changes in the bladder leading to hyperactivity and decreased intervals between contractions following an intravesicular administration of nicotine. In our series, those results were confirmed indirectly following the administration of nAChR antagonists.
There are several limitations to our study. First, we did not evaluate the residual urine volume in the BOO animal model. α3 and α7 nAChR antagonists may reduce the external sphincter resistance and thus, indirectly reduce the bladder contraction pressure. Therefore, we could not exclude the possibility that the reduction in the contraction pressure is due to the decrease of urethral resistance after the administration of nicotinic receptor antagonists. Second, we used just one dosage each for HM and MLC, but these doses may not produce the maximal effect on the bladder of rats. Further studies are warranted to examine the α3 and α7 nAChRs in the urothelium and detrusor muscles and develop drugs targeting these receptors. Such studies would be helpful for regulating detrusor overactivity caused by BOO to control symptoms of OAB.
This study confirmed that the α3 and α7 nAChRs have roles in inducing detrusor hyperactivity in rats subjected to partial BOO. We also confirmed that the α3 and α7 nAChR antagonists regulated this hyperactivity. Further studies are warranted to examine whether detrusor overactivity caused by factors other than obstruction might also be associated with these receptors.