Clinical and Pathological Characteristics of Hard Nodules Resistant to Morcellation During Holmium Laser Enucleation of the Prostate
Article information
Abstract
Purpose:
To identify the clinical and pathological characteristics of hard nodules resistant to morcellation (HNRM) during holmium laser enucleation of the prostate (HoLEP) for benign prostatic hyperplasia (BPH).
Methods:
Between July 2008 and October 2011, 246 patients underwent HoLEP for symptomatic BPH. The first 30 patients were excluded from the analysis due to the learning curve of the procedure. The remaining patients were divided into HNRM (n=29) and non-HNRM groups (n=187), and comparative analysis of the clinical parameters of the two groups was performed. International prostate symptom score analysis and urodynamic studies were performed preoperatively. Histological analysis was performed after hematoxylin and eosin staining and Masson trichrome staining of the HNRM specimens.
Results:
Twenty-nine patients (13.4%) had HNRM. The patients in the HNRM group had significantly higher proportions of advanced age (≥65 years, P=0.029), total prostate volume ≥65 mL (P<0.001), transition zone volume ≥35 mL (P<0.001), serum prostate-specific antigen levels ≥10 ng/mL (P=0.007), and functional urethral length ≥70 mm (P=0.009); larger enucleation weight (P<0.001); longer operation (P=0.001), enucleation (P=0.042), and morcellation times (P<0.001); and higher enucleation ratio (P=0.028) and enucleation efficacy (P=0.001). After adjusting for confounding factors, multivariate logistic regression analysis revealed that age ≥65 years and total prostate volume ≥65 mL were independent risk factors for HNRM. Pathological examination did not reveal any malignant cells, with mainly dense fibrous tissue found in the HNRM.
Conclusions:
HNRM can make morcellation cumbersome and time-consuming, and older patients with larger prostates have a higher incidence of HNRM. However, the histopathology of HNRM revealed mainly fibrotic tissue.
INTRODUCTION
Benign prostatic hyperplasia (BPH) is a common cause of lower urinary tract symptoms (LUTS) and bladder outlet obstruction in elderly men. It is generally accepted that surgery is the standard of care for most cases of symptomatic BPH after failure of medical therapy. Holmium laser enucleation of the prostate (HoLEP) is a well-established, minimally invasive treatment option for men with BPH [1]. There is an increasing amount of evidence supporting the safety and efficacy of HoLEP for BPH when compared to other surgical treatments such as open prostatectomy and transurethral resection of the prostate [2,3]. However, a number of studies have shown that a steep learning curve exists for this operation, thus limiting its widespread use.
In most cases, morcellation can be efficiently and safely performed in patients with prostates of varying sizes [4]. In recent years, this procedure has been improved significantly by the application of mechanical tissue morcellators [5,6]. However, notably, previous studies have reported that the so-called “crazy ball” or “beach ball” effect, which is caused by tissue spheres against the blade of the morcellator, makes morcellation difficult [7,8]. Additionally, El-Hakim and Elhilali [9] reported that small round tissue fragments may persist after morcellation and that these “beach balls” are difficult to morcellate because they dislodge from the morcellator blades. These findings are consistent with our clinical experience.
In the present study, the tissue mentioned above is referred to as hard nodules resistant to morcellation (HNRM). Although previous investigations have shown the existence of HNRM and pointed out that this phenomenon can be cumbersome during morcellation, their impact on the operation efficiency remains unknown, as do their clinical parameters and pathological characteristics. Therefore, this study aimed to identify the clinical and pathological characteristics associated with HNRM.
MATERIALS AND METHODS
Study Population
This study was approved by the Institutional Review Board of Seoul National University Hospital (IRB No. 1111-040-385). A total of 216 consecutive patients who underwent HoLEP for symptomatic BPH between July 2008 and October 2011 were included. Patients with a history of genitourinary malignancy, genitourinary surgery or radiation, interstitial cystitis or bladder pain syndrome, urinary tract infection, or congenital genitourinary anomaly were excluded from the study. Additionally, the first 30 patients were also excluded because of the learning curve period associated with the procedure. The patients were divided into two groups, the HNRM group (n =29) and the non-HNRM group (n=187), based on the need for intraoperative interventions to remove HNRM.
Operative Procedure
All surgical procedures were performed by one surgeon (S.J.O.) according to the technique described previously [10]. In brief, anatomical enucleation was adopted using the three-lobe technique. A 26-Fr resectoscope (Karl Storz, Tuttlingen, Germany) was used for enucleation of the prostate. A 550-μm end-firing laser fiber (SlimLine, Lumenis Ltd., Yokneam, Israel) was engaged with an 80-W holmium neodymium:yttrium-aluminum-garnet laser (VersaPulse Power-Suite, Lumenis Ltd.). The energy power was usually set at 2 J and 40 Hz. Continuous irrigation was applied with normal saline during enucleation and morcellation. The mechanical morcellator used was a VersaCut morcellator (Lumenis Ltd.) through a 0-degree rectangular nephroscope (Karl Storz). If the lump was small, it was washed out through the resectoscope sheath naturally. After complete retrieval of the enucleated prostatic tissue from the bladder, usually a 22-Fr 3-way urethral catheter was inserted for continuous bladder irrigation with normal saline. At the end of the operation, the extracted tissues were weighed after removing the irrigation fluid and sent for pathological analysis.
Definition of HNRM
HNRM was defined as an enucleated prostate lump that was resistant to morcellation and required alternative methods for extracting the tissue from the bladder. The time needed for the alternative method was included in the morcellation time. Some nodules were retrieved using special equipment such as an endoscopic grasper. In some cases, the HNRM were lodged in the morcellator blades and caused morcellator failure. In those cases, electronic transurethral resection was adopted for further fragmentation of the enucleated HNRM. Commonly, the morcellator blades were replaced after eight surgeries.
Clinical Data Collection and Definition of Variables
All patients were evaluated preoperatively by physical examination with digital rectal examination, scoring of subjective symptoms with the International Prostate Symptom Score (IPSS), quality of life (QoL) assessment, laboratory analysis with urinalysis, urine culture (in the presence of pyuria), total serum prostate-specific antigen (PSA), and transrectal prostate ultrasound. Multichannel video urodynamic study (MMS UD 2000, Medical Measurement System, Enschede, The Netherlands) was performed to help identify obstruction and overactivity of the bladder components of the LUTS if necessary. To facilitate the statistical analysis, age ( <65 years vs. ≥65 years), body mass index (<23 vs. kg/m2 ≥23 kg/m2), subtotal voiding symptom score (<5 vs. ≥5), subtotal storage symptom score (<4 vs. ≥4), total prostate volume (TPV; <65 mL vs. ≥65 mL), transition zone volume (<35 mL vs. ≥35 mL), PSA (<10 ng/mL vs. ≥10 ng/mL), and functional urethral length (<70 mm vs. ≥70 mm) were dichotomized. The bladder outlet obstruction index, which is equal to the detrusor pressure at the maximal flow rate (PdetQmax)–2Qmax, was used to evaluate the bladder outlet obstruction [11]. According to the bladder contractility index, bladder contractility can be determined as strong (>150), normal (100–150), and weak (<100) [12]. The IPSS scores were defined as no or mild (0–7) and moderate or severe (≥8), and the QoL scores were defined as mild (0–1) or moderate or severe (≥2) [13]. Operative parameters were obtained from the operation records, and included the retrieved tissue weight, operative time, enucleation time, morcellation time, enucleation ratio (enucleation weight/transitional zone volume), enucleation efficacy (enucleated weight/enucleation time), morcellation efficacy (enucleated weight/morcellation time), enucleation ratio efficacy (enucleation ratio/enucleation time) [14], intraoperative complications, and perioperative outcomes. Complications were assessed according to the Clavien-Dindo classification [15].
Histological Evaluation
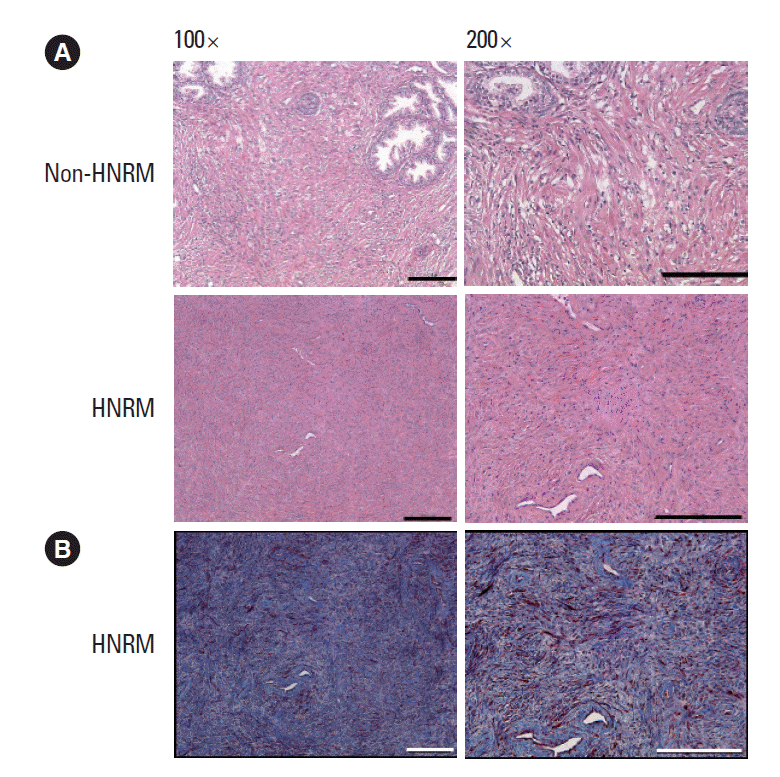
The tissue slices from the prostatectomy specimens were fixed in 10% phosphate buffered formalin, embedded in paraffin, and sectioned and stained with hematoxylin and eosin. Masson trichrome staining was carried out for the HNRM group to distinguish the prostate smooth muscle cells (red) from collagen fibers (blue) and other stromal cell types. Sections were imaged using microscopy (Leica Microsystems, Wetzlar, Germany) at magnifications of ×100 and ×200.
Statistical Methods
The independent t-test or Mann-Whitney U-test was used for comparing the mean differences in continuous variables between the two groups. Pearson chi-square or Fisher exact test was used for binary variables. To identify the independent risk factors for HNRM, we performed univariate and multivariate analyses with logistic regression to estimate the strength of influence of each variable. The odds ratios and 95% confidence intervals were calculated. All P-values were two-sided, and P<0.05 was considered statistically significant. Data were analyzed using IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA).
RESULTS
Baseline Characteristics and Clinical Parameters
Twenty-nine patients (13.4%) were found to have HNRM during morcellation. The basic demographics of the study patients are listed in Table 1. The HNRM group had significantly higher proportions of older age (≥65 years), larger total volume (≥65 mL) and transition zone volume (≥35 mL) of the prostate, higher serum PSA levels (≥10 ng/mL), and longer functional urethral length (≥70 mm) than the non-HNRM group (P<0.05). In addition, the former group had significantly longer operation, enucleation, and morcellation times; larger enucleation weight; higher enucleation ratio and enucleation efficacy; and more energy consumption than the latter (P<0.05). There were no significant differences in the other urodynamic variables (P >0.05). Intraoperative complications occurred in 25 cases, including 5 case of bladder mucosal injury (Clavien-Dindo classification grade I) and 6 cases of prostatic capsular injury (grade I). The complication rates were similar between the two groups (P>0.05). All complications were mild and were managed properly. Additionally, there were no significant differences in the length of hospital stay between the two groups (P>0.05) or in the operative efficiency, including the morcellation efficacy and enucleation ratio efficacy (P>0.05).
The risk factors for HNRM are shown in Table 2. In the univariate logistic regression models, age≥65 years, PSA≥10 ng/mL, TPV≥65 mL, transition zone volume≥35 mL, and functional urethral length≥70 mm were significant risk factors. In the multivariate logistic regression model, age≥65 years and TPV≥65 mL were found to be significantly and independently associated with HNRM. Presence of longer functional urethral length≥70 mm was a borderline significant risk factor in multivariate regression analysis.
Histological Analysis
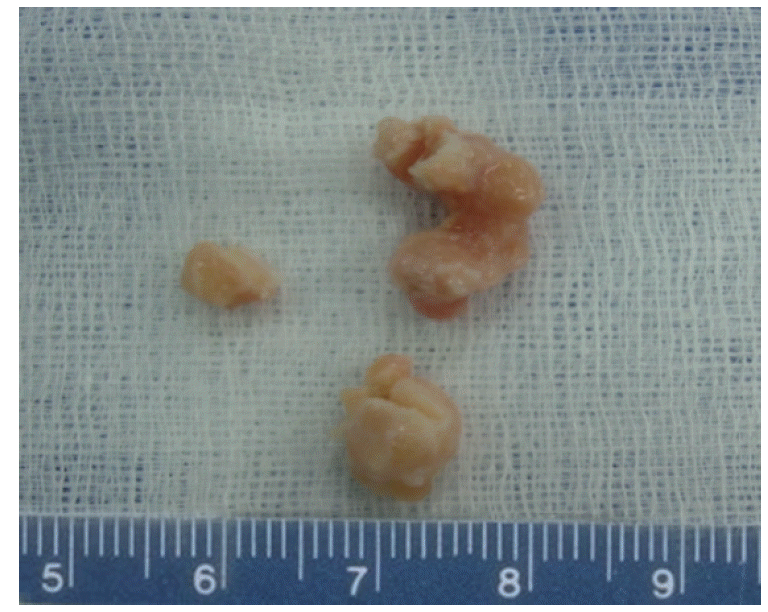
The shapes of the HNRM were irregular, with diameters ranging from 7 to 13 mm, as shown in Fig. 1. Each HNRM was grayish-white with a tough consistency. Microscopically, hematoxylin and eosin staining showed that most nodules were predominantly fibrous proliferations, with only scattered blood vessels and occasional inflammatory cells noted. No malignant cells were observed, and the glandular cells were found to have uniform and basally located, small nuclei. The stromal tissues around the glandular cells were highly proliferated and exhibited a high density of fibers (Fig. 2A). Little calcification was observed. Masson trichrome staining of the HNRM sections showed that the tissue was predominantly and intensely stained blue, containing very little red staining and a few black-stained nuclei (Fig. 2B).
DISCUSSION
Currently, the etiology of hard nodules of BPH is not fully understood. However, prostate ischemia has been proposed to contribute to prostate fibrosis [16]. Kozlowski et al. [17] reported that chronic ischemia resulted in thickening and fibrosis of the prostate in rats, and, similarly, Zarifpour et al. [18] showed that chronic pelvic ischemia caused distinct functional and morphological changes in the prostate in a rat model of arterial endothelial injury, with prostatic tissue from ischemic animals showing an increased deposition of collagen. Moreover, Bauman et al. [19] showed that the proportion of larger bundles of collagen, but not total collagen, was increased in BPH nodules, and concluded that these fibers may play a role in BPH. Berger et al. [20], using contrast-enhanced color Doppler ultrasonography, found that perfusion in the transition zone of the prostate was significantly reduced in men with diabetes or peripheral arterial occlusive disease, compared to in healthy controls. Taken together, this indicates that age-related impairment of blood supply to the lower urinary tract might play a role in the development of BPH and that it may also impact prostate enlargement and/or prostatic smooth muscle tone. In this study, the observed relationship between HNRM and histologic changes in the prostate is difficult to explain, but we speculate that the relation may be due to aging-related chronic ischemia of the lower urinary tract. However, further studies are needed to explore the relationship between fibrotic change and HNRM.
In the present study, clinical parameters were compared between non-HNRM and HNRM groups. Furthermore, the significant predictors related to HNRM were determined, and the histologic features of HNRM were analyzed. The results showed that, compared with patients in the non-HNRM group, the patients in the HNRM group had significantly higher age, larger total and transition zone volumes of the prostate, and higher serum PSA levels, confirming that prostate volume and serum PSA concentration were significantly and positively correlated with advanced age, as previously reported [21].
Our results also indicated that the presence of HNRM could significantly extend the operation, enucleation, and morcellation times. If the core sizes were small, HNRM could be morcellated efficiently or retracted using forceps, whereas if the core sizes were too large to pass through the resectoscope sheath, resection using an electronic resection loop or laser firing for further fragmentation was required in order to allow the HNRM to be extracted through the sheath. As a result, the operation, enucleation, and morcellation times in patients in the HNRM group were significantly longer than in the non-HNRM group. To the best of our knowledge, this is the first study showing HNRM as a hindrance to morcellation, making it cumbersome and time-consuming. Vavassori et al. [7] suggested keeping at least two new, spare sharp blades available in order to avoid the issue of tissue spheres against the sheath of the morcellator; however, it should be noted that the morcellation was not improved by using fresh morcellator blades in the present study.
The patients in the HNRM group showed significant higher enucleation ratio and enucleation efficacy than the patients in non-HNRM group. In a previous study, both the enucleation and morcellation efficacy showed a strong linear correlation with prostate size in all patients [10], implying that HoLEP is more effective for larger prostates. However, this clear linear correlation was not observed in this study. Herein, the morcellation efficacy did not show a significant difference between the groups. The morcellation efficacy is mainly limited by the presence of hard nodules, as hard nodules are generally difficult to grasp and cut with the morcellator device, resulting in the morcellation being cumbersome and time-consuming. On the other hand, patients with HNRM had significantly larger prostate volume than those with non-HNRM in the current study. Consequently, the absolute value of the morcellation time in each patient in the HNRM group was significantly longer. Furthermore, there was no significant difference in the morcellation efficacy between the non-HNRM and HNRM groups as a result of the hard nodules. In addition, in the HNRM group, the patients showed a significant longer functional urethral length than those in the non-HNRM group. This result was consistent with the finding that there was a significant increase in the prostate volume in the HNRM group. Lastly, in this study, the preoperative patient data (Table 1) revealed that the score for each IPSS parameter and the urodynamic parameters were not significantly different in patients with or without HNRM. Taken together, these findings confirm the results of previous studies, suggesting that LUTS do not appear to correlate with the prostate size, PSA level [22], or urinary flow rate [23-25].
The most serious potential complication during HoLEP is bladder perforation, which can result from collapse of the bladder dome down onto the morcellator as a result of inadequate bladder filling during intravesical tissue morcellation. Extended morcellation time increases this risk; therefore, adequate irrigate inflow should be maintained during morcellation. Further, as expected, complications were rare and no significant difference in the complication rate was observed between the groups in this study (Table 1). However, a larger case series is warranted for further evaluation.
Finally, univariate and multivariate logistic regression models were used to calculate the odds ratios of HNRM for potential risk factors (Table 2). Advanced age, larger total and transition zone volumes, higher PSA levels, and longer functional urethral length were found to be individually associated with outcomes in the univariate logistic regression models. Moreover, in the multivariate regression analysis, advanced age and larger TPV were independent risk factor for HNRM. The trend between advanced age and TPV has been previously demonstrated, and in the current study, presence of HNRM appeared to follow the same trend. Histologically, the HNRM were found to consist of fibrotic tissue, and previous in vivo studies have demonstrated the evolution of stromal fibrosis with progression of BPH [26].
This study has some potential limitations. First, this was a retrospective study and unidentified confounding factors beyond our control may have been present. Furthermore, our study cohort was collected from a single institution with a relatively limited number of patients. Therefore, further studies on the prediction of HNRM before surgery are expected in the near future, for example using computed tomography or transrectal prostatic ultrasound analysis. A more thorough evaluation in a larger series of patients with HNRM is necessary to confirm the results.
In conclusion, our results showed that the presence of HNRM can make morcellation cumbersome and time-consuming. The proportion of HNRM increased in patients with advanced age ≥65 years and with a TPV≥65 mL. Moreover, histologically, the HNRM were found to mainly be composed of fibrotic tissue.
Notes
Research Ethics
This study was approved by the Institutional Review Board of Seoul National University Hospital (IRB No. 1111-040-385).
Conflict of Interest
No potential conflict of interest relevant to this article was reported.