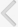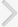INTRODUCTION
The high prevalence of lower urinary tract symptoms (LUTS) in the elderly is caused by age-related changes to the pelvic floor and lower urinary tract anatomy and function [1]. Overactive bladder (OAB) is one of the most annoying LUTS; it is characterized by urinary urgency with or without urge incontinence, usually associated with frequency and nocturia. Indeed, urinary urgency is the chief element of an OAB diagnosis [1]. Considering the extent of symptoms, drug side-effect profiles, incidence of coexisting diseases and existing drug regimens, OAB treatment must be personalized for each patient [1]. Muscarinic antagonists, the most commonly used remedy for OAB, have limited efficacy and dose-related side effects. Mirabegron, a selective androgen receptor (AR) 3 agonist, has been recently approved for OAB treatment in spite of having unresolved issues of efficacy and safety in weak elderly patients or upon prolonged therapy [2,3]. Combinations of therapeutic agents with different mechanisms of action, such as a muscarinic antagonist and an AR 3 agonist, offer new avenues for OAB therapy [1]. Therefore, there is growing demand for effective OAB therapeutics, either as alternatives to or in combination with existing drugs. LUTS are twice as frequent in women as in men. Estrogen deficiency caused by menopause is known to be involved in urinary dysfunctions, such as an OAB, urinary incontinence, and detrusor underactivity [4]. Although results from previous studies are controversial, they suggest that local application of estrogen through the vaginal route had a therapeutic effect on OAB [4,5]. Moreover, a combination of muscarinic antagonists and topical vaginal estrogen has been shown to have a synergistic effect on OAB in postmenopausal women [6]. However, the mechanism of action of systemic estrogen replacement and its effect on LUTS are still unclear.
Potassium (K+) channels are involved in the regulation of nerve and muscle cell excitability in various tissues, including the urinary bladder, and may be regarded as attractive targets for OAB treatment. The calcium-activated potassium (KCa) channels are found in detrusor smooth muscle cells [7-10]. KCa channels are defined based on their single channel conductance as follows: large (BK), intermediate (IK), and small (SK1–SK3) channels. A recent review by Petkov [11] suggested that BK is the most important K+ channel in the regulation of the human urinary bladder smooth muscle (UBSM) excitability. BK channels are activated by changes in membrane depolarization and increases in intracellular Ca2+. They are composed of either a homotetramer of pore-forming خ±-subunits alone, or four خ±-subunits combined with tissue-specific regulatory خ²-subunits [11]. Since the pore-forming خ±-subunit of the BK channel is encoded by a single Slo gene, alternative splicing of Slo may explain the channel’s functional diversity [11,12]. Several studies have shown that estrogen increases the BK channel’s open probability, and hence its activity in various smooth muscle cells [13-15]. Additionally, Afeli et al. [16] demonstrated that SK channels were involved in the regulation of spontaneous and nerve-stimulated contraction of human UBSM cells. Therefore, a drug related to KCa channels could be a potential new candidate for OAB treatment, especially in women. At present, there is insufficient knowledge about the effect of estrogen on BK channel gene and protein expression in the whole urinary bladder because pharmacological studies were conducted on UBSM cells only.
The aim of the present study was to examine the effect of 17خ²-estradiol on the number of KCa channel proteins expressed in the bladder of female ovariectomized rats.
MATERIALS AND METHODS
Bilateral Ovariectomy and Tissue Preparation All animal experiments were approved by the Animal Subjective Committee of Kyungpook National University. Ten-week-old female Sprague-Dawley rats (body weight [BW], 200–220 g; n=34) were randomly divided into 3 groups: sham-operated controls (n=11), bilateral ovariectomized rats (n=11), and bilateral ovariectomized rats with estrogen replacement (n=12). Bilateral ovariectomy was performed aseptically through a midline abdominal approach under general anesthesia with enflurane. Animals were treated with antibiotic (150 mg/kg/day ampicillin) for 3 days. Immediately after operation, estrogen replacement was performed by subcutaneous injection with 50 خ¼g/kg of 17خ²-estradiol benzoate (Sigma Chemical Co., St. Louis, MO, USA) every other day for 2 weeks [17]. Rats were sacrificed 2 weeks after ovariectomy and the whole bladder was isolated and divided in two. One half was immediately minced for protein extraction. The other was quickly frozen in liquid nitrogen, and stored at −70آ°C for RNA extraction.
Reverse Transcription Polymerase Chain Reaction
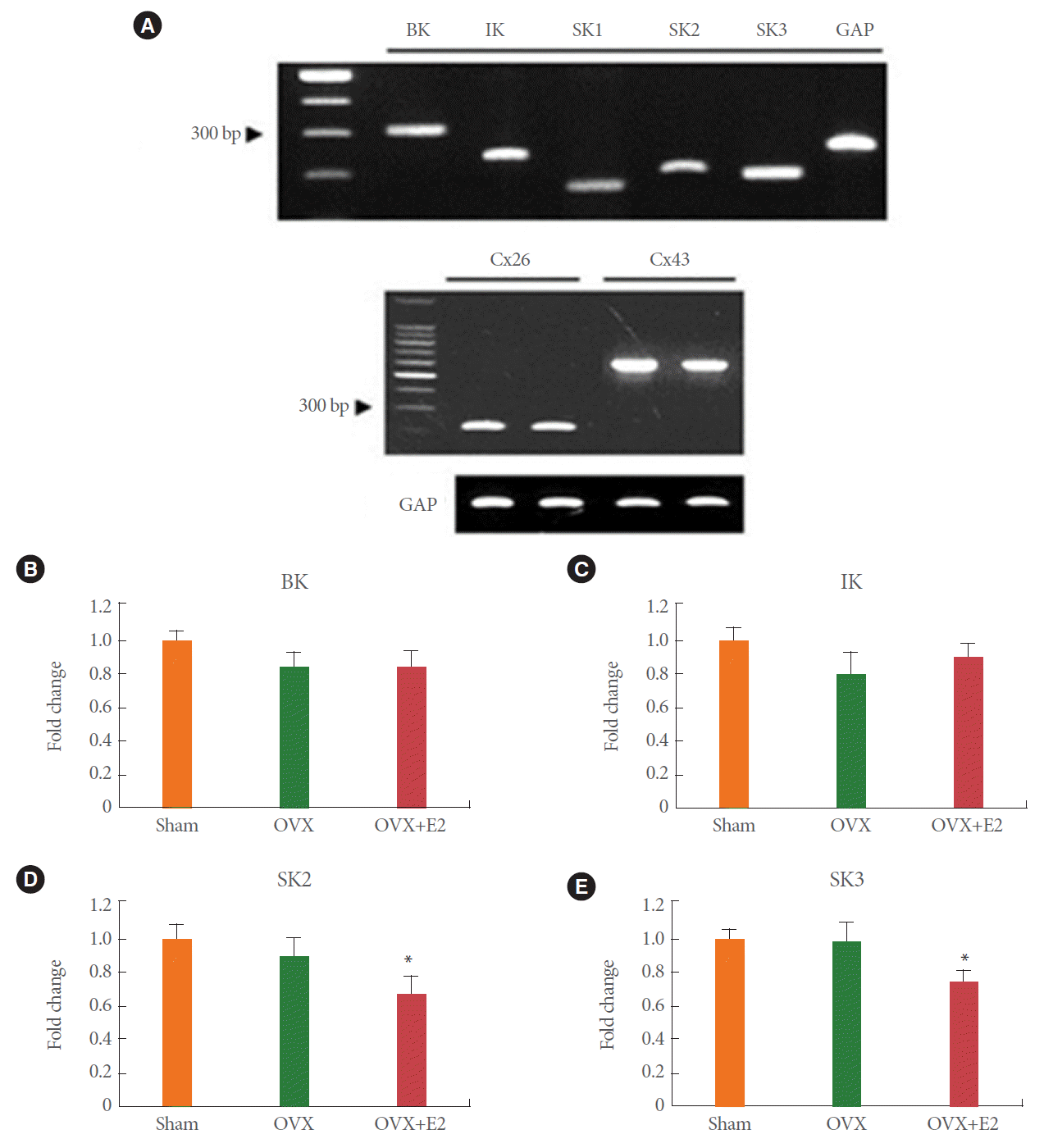
Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and cDNA was synthesized with M-MLV reverse transcriptase (Promega, Madison, WI, USA). cDNA was amplified with Taq polymerase (Finnzymes, Espoo, Finland) in a DNA thermal cycler (MJ research, Watertown, MA, USA). The following cycling conditions were applied: 30 cycles of 1 minute at 95آ°C, 45 seconds at the annealing temperature, and 1 minute at 72آ°C. Annealing temperatures were 50آ°C for connexin 43 (Cx43); 53آ°C for SK3; 55آ°C for BK, SK1, SK2, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH); 57آ°C for IK; and 59آ°C for connexin 26 (Cx26). One-tenth of each polymerase chain reaction (PCR) product was resolved on a 1% agarose gel containing 0.5 خ¼g/mL of ethidium bromide, and quantified using Quantity One 1-D image analysis software (Bio-Rad, Hercules, CA, USA). Primers for IK (GenBank AF149250), Cx26 (GenBank X51615), Cx43 (GenBank: M19317), and GAPDH (GenBank NM_017008) were designed using Primer 3-software [18]. BK [19] and SK [20] primer sequences were designed on the basis of previous reports. The primer sequences were as follows: BK (312 bp), 5خ„-GGCTGGAAGTGAATTCTGTAG-3خ„ (forward) and 5خ„-TGAGTAAGTAGACACATTCCC-3خ„ (reverse); IK (233 bp), 5خ„-CTTGGGTGCTGTCTGTGG-3خ„ (forward) and 5خ„-GTGTTTCTCCGCCTTGTTG-3خ„ (reverse); SK1 (159 bp), 5خ„-CAGGCCCAGCAGGAGGAGTT-3خ„ (forward) and 5خ„-GGCGGCTGTGGTCAGGTG-3خ„ (reverse); SK2 (190 bp), 5خ„-TCCGACTTAAATGAAAGGAG-3خ„ (forward) and 5خ„-GCTCAGCATTGTAGGTGAC-3خ„ (reverse); SK3 (182 bp), 5خ„-GTGCACAACTTCATGATGGA-3خ„ (forward) and 5خ„-TTGACACCCCTCAGTTGG-3خ„ (reverse); Cx26 (220 bp), 5خ„-GCCCCCAGTTAAGGGTAAAG-3خ„ (forward) and 5خ„-CCATGCTCACATCACAAACC-3خ„ (reverse); Cx43 (627 bp), 5خ„-GACTGCTTCCTCTCACGTC-3خ„ (forward) and 5خ„-TAGGTGCATGTTCTGCAAGC-3خ„ (reverse); and GAPDH (230 bp), 5خ„-ATCAAATGGGGTGATGCTGGTGCTG-3خ„ (forward) and 5خ„-CAGGTTTCTCCAGGCGGCATGTCAG-3خ„ (reverse). A single PCR product of the expected size was detected for each sample. The intensity of each band was normalized to that of GAPDH.
Western Blot Analysis
Each bladder sample was homogenized in cold lysis buffer (150mM NaCl, 25mM Tris-HCl, pH 7.4, 1% Triton X-100, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 1% Nonidet P-40) containing a protease inhibitor cocktail (Roche, Basel, Switzerland). Protein from each sample (40 خ¼g) was separated by 10% SDS-polyacrylamide gel electrophoresis, transferred onto a nitrocellulose membrane, and incubated overnight at 4آ°C with primary antibodies (1:500) against KCa channels (Alomone Labs, Jerusalem, Israel), Cx23 and Cx43 (both Santa Cruz Biotechnology, Dallas, TX, USA). Membranes were then washed and incubated with a secondary antibody conjugated to peroxidase (1:2,000, Santa Cruz Biotechnology). Immunoreactive signals were visualized on autoradiography film after an enhanced chemiluminescence reaction (Amersham Biosciences, Buckinghamshire, UK). Band intensities were quantified using a molecular imager and Quantity One 1-D image analysis software (Bio-Rad). Equal protein loading was confirmed by subsequently probing the membranes with an antibody against خ²-actin (1:3,000; Santa Cruz Biotechnology).
Data Analysis
Data are presented as meansآ±standard error of the mean. In each sample, mRNA expression was normalized to GAPDH and plotted as fold induction relative to the average of the control group. Protein levels were normalized to خ²-actin. Differences between groups were evaluated using Student t-test, Mann-Whitney test, or Kruskal-Wallis one-way analysis of variance with Tukey test for multiple comparison. P<0.05 was considered significant.
RESULTS
Body and Uterine Weight
As previously reported [21], rats from the ovariectomy alone group gained more weight than those from the other groups. Baseline and final BWs of rats in the 3 groups were as follows: sham-operated control (n=11) baseline BW, 191.4آ±15.3 g; final BW, 211.4آ±14.0 g; ovariectomy alone (n=11) baseline BW, 201.7آ±15.3 g; final BW, 235.4آ±17.4 g; ovariectomy plus estrogen replacement (n=12) baseline BW, 191.4آ±18.2 g; final BW 196.4آ±17.3 g. Uterine weight decreased significantly in the ovariectomy alone group compared with the other groups, as previously reported [21].
mRNA Expression of KCa and Gap Junction Channels in Rat Bladder
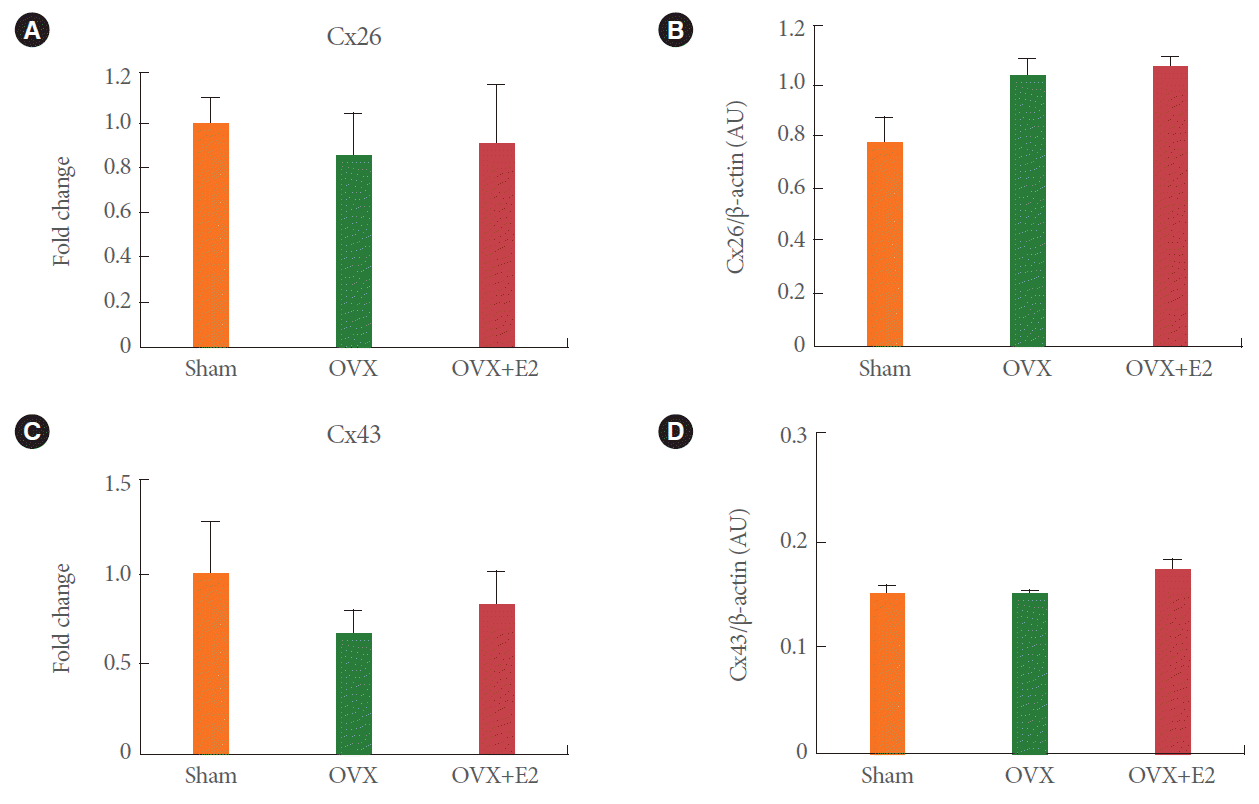
Semiquantitative PCR measurements revealed the presence of mRNAs for all subtypes of KCa channels, Cx26 and Cx43 (Fig. 1A). In the absence of any detectable SK1 channel protein, SK1 expression was not determined. Expression of BK, IK, Cx26, and Cx43 in the bladder of bilaterally ovariectomized rats did not differ significantly from that in the control, or estrogen replacement groups (Figs. 1B, C, 2A, C). At the same time, SK2 and SK3 expression decreased significantly after estrogen replacement, but not after ovariectomy alone (Fig. 1D, E).
BK and SK3 Protein Levels Decreased in Ovariectomized Rats Upon Estrogen Replacement
Protein levels of BK in the bladder of bilaterally ovariectomized rats increased by 1.5 folds compared with those in the shamoperated controls (Fig. 3A, B), but were restored by estrogen replacement (Fig. 3B). In addition, SK3 protein levels were unchanged by ovariectomy alone, but they decreased to 75% of control levels upon 17خ²-estradiol treatment for 14 days after surgery (Fig. 3E). Levels of IK, SK2, Cx26, and Cx43 did not change significantly under any condition (Figs. 2B, D, 3C, D).
DISCUSSION
The present study shows that the increase in BK protein levels in rat bladder caused by ovariectomy can be reversed by concomitant estrogen administration. In addition, we found that SK3 mRNA and protein levels decreased significantly upon 17خ²-estradiol treatment after surgery, but were not affected by the procedure itself.
The absence of any significant differences between the BKخ±-subunits’ mRNA levels in the three groups coincides with the results from a previous study on rat aortas [22]. The authors found that both خ±-and خ²-subunit mRNA levels of vascular BK channels were unaffected by ovariectomy alone or 17خ²-estradiol replacement. Various studies on smooth muscles have reported that both BK channel activity and smooth muscle relaxation were augmented by estrogen [13-15]. Thus, estrogen deficiency caused by ovariectomy would be expected to lower BK mRNA and protein levels. However, unexpectedly, the 1.5-fold BK protein increase was not accompanied by a change in mRNA expression. A possible explanation for this mismatch is the occurrence of posttranscriptional regulation by estrogen-regulated microRNAs (miRNAs). miRNAs are small, single-stranded, noncoding RNA molecules, whose main roles in posttranscriptional regulation are translational repression or mRNA degradation [23,24]. Vasudevan et al. [23] documented that miRNA increased the translation of target mRNAs during cell cycle arrest. A recent review by Klinge [24] has provided a comprehensive review of estrogen-regulated miRNAs and their role in modulating estrogen receptor expression and function. A recent study by Pietrzykowski et al. [25] has shown that the stability of BK mRNA splice variants is regulated posttranscriptionally by miRNAs, such as miR-9. In addition, a very recent study on osteosarcoma cells [26] has shown that miR-9 increases upon 17خ²-estradiol treatment. Thus, it is possible that estrogen-related miRNAs down-regulate BK translation and/or BK mRNA stability. It may also be possible that miRNAs affect BK channel expression by regulating the stability and translation of estrogen receptor mRNAs. As most studies on estrogen-regulated miRNAs were done in the context of breast cancer, there is limited data on its role in bladder pathophysiology. Similarly, studies on miRNAs and BK mRNA stability are also scarce. Therefore, further work is needed to investigate the hypotheses mentioned above.
Changes to intracellular trafficking could also explain the difference between BK mRNA and protein levels. A previous study by Eghbali et al. [27] demonstrated that BK channel protein accumulated in the perinuclear organelles of mouse myometrium under the control of sex hormones.
A previous study by Zhu et al. [12] revealed that alternative splicing of the Slo gene may be related to the lowering of BKخ±-subunit protein levels by 17خ²-estradiol treatment in the ovariectomized rat myometrium. Therefore, one possible explanation for the rise in BK protein levels under ovariectomy may be linked to the regulation of Slo gene splicing by 17خ²-estradiol in UBSM cells. Another possibility relates to changes in the stoichiometry of BK channel subunits. In reproductive arterial vascular smooth muscle of nonpregnant sheep, Nagar et al. [15] provided evidence that 17خ²-estradiol regulated the expression of the BKخ²1 subunit and altered the function and estrogen responsiveness of the BK channel by modifying the stoichiometry of the خ±:خ²1 subunit. A previous study by Valverde et al. [28] suggested that the coassembly of BK channel خ±- and خ²-subunits in different tissues could explain its functional diversity upon estrogen modulation. Four خ²-subunits combine with the pore forming خ±-subunit tetramer to regulate BK channel function in various tissues [7,28]. The خ²-subunits are classified into 4 subtypes, خ²1 to خ²4. The خ²1-subunits are known to be major accessory subunits in smooth muscles and are abundant in UBSM cells [7,28]. In addition, Chen and Petkov [8] described the neuronal-specific خ²4-subunits in rat and mouse UBSM cells suggesting that they may have a role as accessory subunits and may modulate BK function in UBSM cells. Moreover, other researchers reported that, unlike خ²1-subunits, خ²4-subunits decreased Ca2+ sensitivity and slowed the gating kinetics of BK channels [29,30]. Finally, a recent study by Yan and Aldrich [31] has shown the existence of four new accessory subunits (خ³1 to خ³4), revealing the possibility of voltage-dependent BK channel activation and tissue-specific mRNA expression. Although we are unable to confirm the exact changes, our findings suggest that 17خ²-estradiol treatment may alter the composition of BKخ± and BKخ²1/BKخ²4.
As reported previously in human whole bladder [15], we were able to detect the IK and SK channel proteins. Of these, only SK3 mRNA and protein levels were shown to decrease significantly upon 17خ²-estradiol treatment after ovariectomy. These findings are consistent with previous studies [15,32], whereby SK3 was shown to be the main IK/SK channel subtype in UBSM cells. Taken together, our findings indicate that SK3 channels may be an important modulator of UBSM excitability under estrogen control.
We also measured connexin Cx26 and Cx43 mRNA and protein levels in the bladder. Gap junctions are known to stimulate detrusor excitability and contractility in certain conditions such as in response to spinal transection and bladder outlet obstruction [33,34]. However, as reported in a recent study [35], connexin mRNA and protein levels failed to show any significant difference between the three groups (control, ovariectomy, and ovariectomy plus estrogen replacement).
In conclusion, the present study demonstrates that the protein levels of the BKخ±-subunit increase upon estrogen deficiency caused by ovariectomy, but can be reversed by 17خ²-stradiol treatment. In addition, protein levels of the SK3 channel decrease only upon 17خ²-estradiol treatment, suggesting that SK3 is the principal IK/SK channel subtype in rat UBSM cells to be under the control of estrogen. These findings may underlie why some estrogen replacement treatments targeted at improving LUTS fail in postmenopausal women.