Neuroprotective Effects of Bone Marrow Stromal Cell Transplantation in Combination With Treadmill Exercise Following Traumatic Brain Injury
Article information
Abstract
Purpose:
Traumatic brain injury (TBI) causes cognitive impairments, motor deficits, and neuropsychiatric/behavioral deficits problems. Transplantation of bone marrow stromal cells (BMSCs) facilitates functional recovery from brain insults. Treadmill exercise increases neurogenesis and inhibits apoptosis. In this study, we investigated the effects of BMSC transplantation in combination with treadmill exercise on memory function, by evaluating its effect on neurogenesis and apoptosis in the hippocampus following TBI.
Methods:
TBI was induced using an electromagnetic-controlled cortical impact device. BMSCs were transplanted into both sides of traumatic scar region 1 week after TBI induction. One week after transplantation of BMSCs, the rats in the exercise groups were trained to run on a treadmill for 30 minutes once daily for 28 days. Step-down avoidance task and radial 8-arm maze test were conducted. Levels of 5-bromo-2ʹ-deoxyuridine and caspase-3 were evaluated using immunohistochemistry. Western blot was used to evaluate the expression of brain-derived neurotrophic factor (BDNF), tyrosine kinase B (TrkB), total-extracellular signal-regulated kinase 1 and 2 (t-ERK1/2), phosphorylated-ERK1/2 (p-ERK1/2), Bcl-2, and Bax.
Results:
TBI deteriorated memory function, suppressed neurogenesis, and accelerated apoptosis in the hippocampus. Treadmill exercise and BMSC transplantation independently improved memory function by increasing neurogenesis with suppression of apoptosis through the BDNF-ERK pathway in the TBI-induced rats. Combination of BMSC transplantation with treadmill exercise showed additional enhancement of neurogenesis and suppression of apoptosis in the hippocampus.
Conclusions:
The present study shows that treadmill exercise may aid the therapeutic effect of BMSC transplantation on TBI in rats.
INTRODUCTION
Traumatic brain injury (TBI) is one of the leading causes of neurological deficits, which result in cognitive impairments, motor deficits, and neuropsychiatric/behavioral deficits problems [1]. Various types of brain insults induce loss of neural precursor cells and reduce the number of newly generated neurons. These changes can result in deterioration of memory function [2] and inhibit long-term potentiation [3]. Neuronal loss has been suggested as the mechanism underlying the development of hippocampus-dependent cognitive deficits in TBI [4,5].
Brain-derived neurotrophic factor (BDNF) acts via receptor tyrosine kinase B (TrkB), and the interaction of BDNF with TrkB is implicated in the synaptic plasticity, learning process, and neurogenesis [6,7]. BDNF activates TrkB signaling and its downstream effectors, including extracellular signal-regulated kinase (ERK). ERK 1 and 2 (ERK1/2) cascade promotes transcription of genes involving in the synapse formation [8].
TBI has been shown to cause apoptotic cell death in hippocampal neurons [4,9]. The members of the B-cell lymphoma 2 (Bcl-2) family and a class of cysteine proteases known as caspases are involved in the process of apoptotic cell death [10,11]. The Bcl-2 family is divided into antiapoptotic proteins and pro-apoptotic proteins. Anti-apoptotic Bcl-2 protects against cell death by regulating apoptotic pathways. Pro-apoptotic Bax expression is enhanced during apoptosis, resulting in acceleration of cell death [10]. Decreased Bcl-2 immunoreactivity and increased Bax immunoreactivity were observed in the injured neurons after TBI [4] and global ischemia [12]. The most well-known member of the caspase family, caspase-3, plays a key role in apoptosis [11], and acts downstream of the Bax/Bcl-2 pathway [13].
Bone marrow stromal cells (BMSCs), also known as mesenchymal stem cells, are multipotent nonhematopoietic stem cells [14]. Transplantation of BMSCs facilitates regeneration of injured axons, and promotes functional recovery following central nervous system injuries via the differentiation of BMSCs into neurons [15-17]. In addition, treadmill exercise is also known to increase neurogenesis through enhancing neurotrophic factors, such as BDNF [7,18].
Transplantation of stromal cells has been used as one of the effective strategies for the treatment of TBI. However, the effects of treadmill exercise on BMSCs therapy in TBI have not been evaluated. In the present study, we investigated whether treadmill exercise potentiated the restorative effects of BMSC transplantation in rats with TBI-induced memory impairment, by evaluating their combined effects on neurogenesis and apoptosis in the hippocampus.
MATERIALS AND METHODS
Cell Culture
BMSCs were obtained from the femoral and tibial bones of 4-week-old rats, as previously described method [15]. Cells were cultured using α-modified Eagle’s medium (α-MEM) with 5% CO2 at 37°C for 3 days. Subsequently, 10 μL of BMSCs containing 105 cells were injected into both sides of the traumatic scar region 1 week after TBI induction.
Animals and Treatments
Male Sprague-Dawley rats weighing 200±10 g (8 weeks) were purchased from the Orient Co. (Seoul, Korea). Experiments were performed in accordance with the guidelines of the Korean Academy of Medical Sciences and the animal care guidelines of the National Institutes of Health. The rats were divided into six groups (n=10, each group): (1) control group, (2) treadmill exercise group, (3) TBI-induced group, (4) TBI-induced and treadmill exercise group, (5) TBI-induced and BMSC transplantation group, and (6) TBI-induced and BMSC transplantation with exercise group. One hour before starting treadmill running, the rats were injected subcutaneously with 50 mg/kg of 5-bromo-2ʹ-deoxyuridine (BrdU, 50 mg/kg; Sigma Chemical Co., St. Louis, MO, USA), once a day for 4 consecutive days.
Induction of TBI
TBI was induced as previously described method [4,9]. The rats were anesthetized with Zoletil 50 (10 mg/kg, intraperitoneally; Virbac Laboratories, Carros, France). Contusion injury was created using an electromagnetic contusion device (Impact One, Stereotaxic Impactor; MyNeurolab, St. Louis, MO, USA) with a sterile stainless steel impactor tip (3.0 mm diameter) at a velocity of 5.00 m/sec. The rats in the control group and in the treadmill exercise group were treated in an identical manner, except that no brain contusion was simulated.
Treadmill Exercise Protocol
One week after BMSC transplantation, the rats in the exercise groups were trained to run on a treadmill for 30 minutes, once daily for 28 days. Exercise load consisted of running at a speed of 2 m/min for the first 5 minutes, 5 m/min for the next 5 minutes, and then at a speed of 8 m/min for the last 20 minutes, at an inclination of 0°.
Step-Down Avoidance Task
Step-down avoidance task was used to evaluate short-term memory, as previously described method [4,19]. The rats were trained in a step-down avoidance task 42 days after TBI induction. In the training session, the animals received a scrambled foot shock of 0.5 mA for 2 seconds immediately upon stepping down. Two hours after training, the latency, defined as the interval between the rats stepping down and placing all four paws on the grid, was determined. Latency over 180 seconds is reported as 180 seconds.
Radial 8-Arm Maze Test
A radial 8-arm maze test was conducted for the evaluation of spatial learning ability, as previously described method [7,20]. The rats were trained three times before the test. After the training sessions, the rats were deprived of water for 24 hours. Subsequently, they were allowed to explore the maze to find the water and given 5 minutes to drink it. The test was conducted 41 days after TBI induction, and the test was terminated when the rats found water in all eight arms or when over 6 minutes had elapsed. Re-entry into the previously visited arms was counted as the error. The number of correct choices before the first error was also counted.
Tissue Preparation
The animals were sacrificed immediately after determining the latency in the step-down avoidance task. The rats were anesthetized using Zoletil 50 (10 mg/kg, intraperitoneally, Virbac Laboratories), followed by transcardial perfusion of 0.05M phosphate-buffered saline, and fixed using 4% paraformaldehyde in 0.5M phosphate buffer (pH 7.4). A freezing microtome (Leica, Nussloch, Germany) was used to prepare 40-μm thick coronal sections.
BrdU Immunohistochemistry
BrdU-specific immunohistochemistry was performed as previously described method [7,19]. The sections were incubated overnight at 4°C with BrdU-specific mouse monoclonal antibody (1:600; Roche, Mannheim, Germany). Next, the sections were incubated with a biotinylated mouse secondary antibody (1:200; Vector Laboratories, Burlingame, CA, USA) for 1 hour. For visualization, the sections were incubated in 50 mM Tris-HCl (pH 7.6) containing 0.02% diaminobenzidine tetrahydrochloride (DAB), 40 mg/mL nickel chloride, and 0.03% H2O2 for 5 minutes. Differentiation of BrdU-positive cells was confirmed on the same section using a mouse antineuronal nuclei antibody (1:300; Chemicon International, Temecula, CA, USA).
Caspase-3 Immunohistochemistry
Caspase-3 immunohistochemistry was performed as previously described method [4,20]. The sections were incubated overnight with a rabbit anti–caspase-3 antibody (1:500; Santa Cruz Biotechnology, Dallas, TX, USA), and then incubated for another 1 hour with the biotinylated rabbit secondary antibody. The bound secondary antibody was then amplified using a Vector Elite ABC kit (Vector Laboratories). The antibody-biotinavidin-peroxidase complex was visualized using 0.02% DAB.
Western Blot Analysis
For the detection of the levels of BDNF, TrkB, total-ERK1/2 (t-ERK1/2), phosphorylated-ERK1/2 (p-ERK1/2), Bax, and Bcl-2, Western blot analysis was conducted as previously described method [4,20]. Hippocampal tissues were lysed in ice-cold lysate buffer. Proteins (40 μg) were separated on sodium dodecyl sulfate-polyacrylamide gels and transferred onto a nitrocellulose membrane (Amersham Pharmacia Biotechnology, Piscataway, NJ, USA). Mouse antiactin (1:500; Santa Cruz Biotechnology), rabbit anti-BDNF, anti-TrkB, anti-t-ERK1/2, anti-p-ERK1/2, anti-Bax, anti-Bcl-2 antibodies (1:1,000; Santa Cruz Biotechnology) were used as primary antibodies. Horseradish peroxidase-conjugated anti-mouse antibody for actin and antirabbit antibodies for BDNF, TrkB, t-ERK1/2, p-ERK1/2, and Bax, Bcl-2 were used as secondary antibodies. Protein bands were detected using the enhanced chemiluminescence detection system (Santa Cruz Biotechnology).
Statistical Analysis
Data were analyzed using IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA). Results were expressed as the mean ±standard error of the mean. One-way analysis of variance with Duncan post hoc test was used for comparison among the groups. P<0.05 was considered statistically significant.
RESULTS
Effects of BMSC Transplantation and Treadmill Exercise on Short-Term Memory
Latency was significantly decreased by TBI induction. Treadmill exercise and BMSC transplantation independently increased the latency in the TBI-induced rats. Combination of BMSC transplantation with treadmill exercise increased the latency further, although the increase was not statistically significant (Fig. 1).
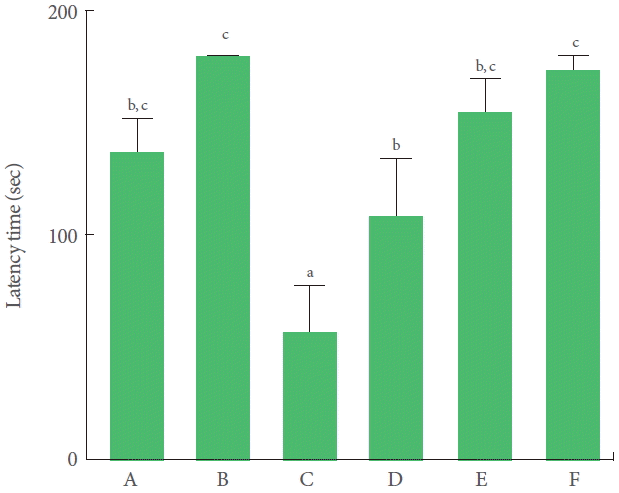
Effects of transplantation of bone marrow stromal cells (BMSCs) and treadmill exercise on short-term memory. A, control group; B, treadmill exercise group; C, traumatic brain injury (TBI)-induced group; D, TBI-induced and treadmill exercise group; E, TBI-induced and BMSC transplantation group; F, TBI-induced and BMSC transplantation with treadmill exercise group. The letters a–c denote statistically significant differences, P<0.05.
Effects of BMSC Transplantation and Treadmill Exercise on Spatial Learning Ability
On induction of TBI, the number of correct choices in the radial 8-arm maze test was significantly decreased and the number of errors was significantly increased. Treadmill exercise and BMSC transplantation independently increased the number of correct choices and decreased the number of errors in the TBI-induced rats. Combining BMSC transplantation with treadmill exercise had a potentiating effect on the number of correct choices; however, the increase in the number of correct choices did not reach statistical significance. In addition, there was no change in the number of errors in the combination protocol (Fig. 2).
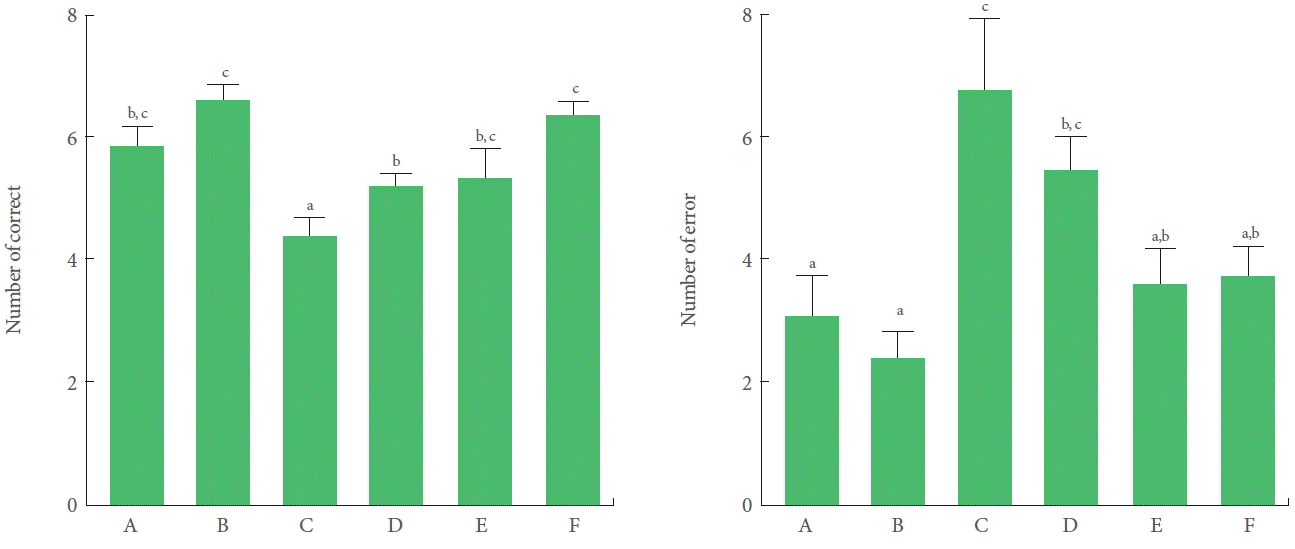
Effects of transplantation of bone marrow stromal cells (BMSCs) and treadmill exercise on spatial learning ability. A, control group; B, treadmill exercise group; C, traumatic brain injury (TBI)-induced group; D, TBI-induced and treadmill exercise group; E, TBI-induced and BMSC transplantation group; F, TBI-induced and BMSC transplantation with treadmill exercise group. The letters a–c denote statistically significant differences, P<0.05.
Effects of BMSC Transplantation and Treadmill Exercise on Neurogenesis in the Hippocampal Dentate Gyrus
The number of BrdU-positive cells in the hippocampal dentate gyrus was significantly decreased by TBI induction. Treadmill exercise and BMSC transplantation independently increased the number of BrdU-positive cells in the TBI-induced rats, though there was no statistical significance. Combination of BMSC transplantation with treadmill exercise significantly increased the number of BrdU-positive cells (Fig. 3).
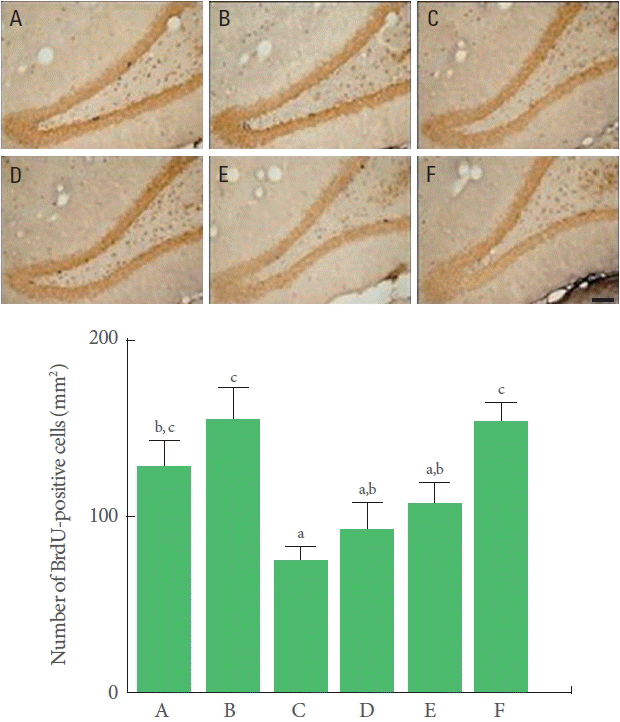
Effects of transplantation of bone marrow stromal cells (BMSCs) and treadmill exercise on neurogenesis. Upper panel: Photomicrographs of 5-bromo-2ʹ-deoxyuridine (BrdU)-positive cells. The scale bar represents 200 μm. Lower panel: Number of BrdU-positive cells in each group. A, control group; B, treadmill exercise group; C, traumatic brain injury (TBI)-induced group; D, TBI-induced and treadmill exercise group; E, TBI-induced and BMSC transplantation group; F, TBI-induced and BMSC transplantation with treadmill exercise group. The letters a–c denote statistically significant differences, P<0.05.
Effects of BMSC Transplantation and Treadmill Exercise on Caspase-3 Expression in the Hippocampal Dentate Gyrus
The number of caspase-3-positive cells in the hippocampal dentate gyrus was significantly increased by TBI induction. Treadmill exercise and BMSC transplantation independently decreased the number of caspase-3-positive cells in the TBI-induced rats. Combination of BMSC transplantation with treadmill exercise significantly decreased the number of caspase- 3-positive cells (Fig. 4).
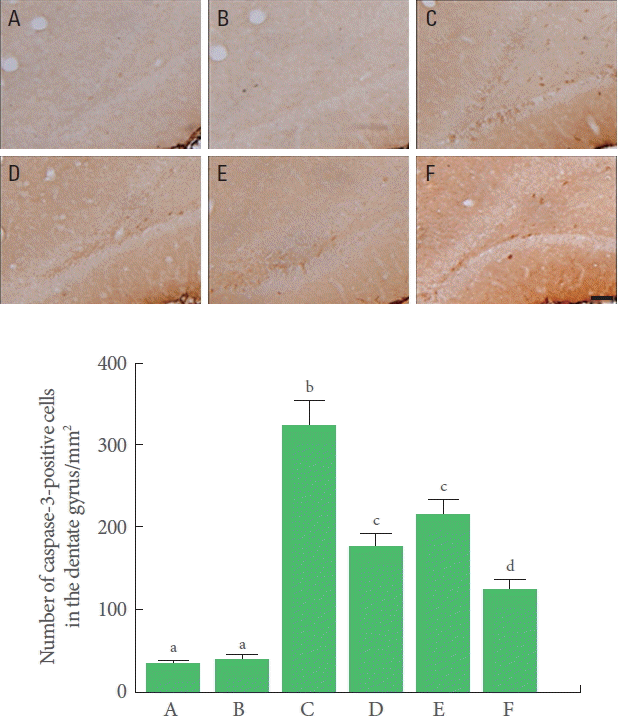
Effects of transplantation of bone marrow stromal cells (BMSCs) and treadmill exercise on caspase-3 expression. Upper panel: Photomicrographs of caspase-3-positive cells. The scale bar represents 200 μm. Lower panel: Number of caspase-3-positive cells in each group. A, control group; B, treadmill exercise group; C, traumatic brain injury (TBI)-induced group; D, TBIinduced and treadmill exercise group; E, TBI-induced and BMSC transplantation group; F, TBI-induced and BMSC transplantation with treadmill exercise group. The letters a–d denote statistically significant differences, P<0.05.
Effects of BMSC Transplantation and Treadmill Exercise on the BDNF-ERK Signaling Pathway
BDNF and TrkB expression in the hippocampus was significantly suppressed by TBI induction. Treadmill exercise and BMSC transplantation independently elevated BDNF and TrkB expression in the TBI-induced rats. Combination of BMSC transplantation with treadmill exercise significantly increased BDNF expression, but had no potentiating effect on TrkB expression. The ratio of p-ERK to t-ERK in the hippocampus was significantly decreased by TBI induction. Treadmill exercise and BMSC transplantation independently increased this ratio in the TBI-induced rats. Combination of BMSC transplantation with treadmill exercise had no effect on this ratio (Fig. 5).
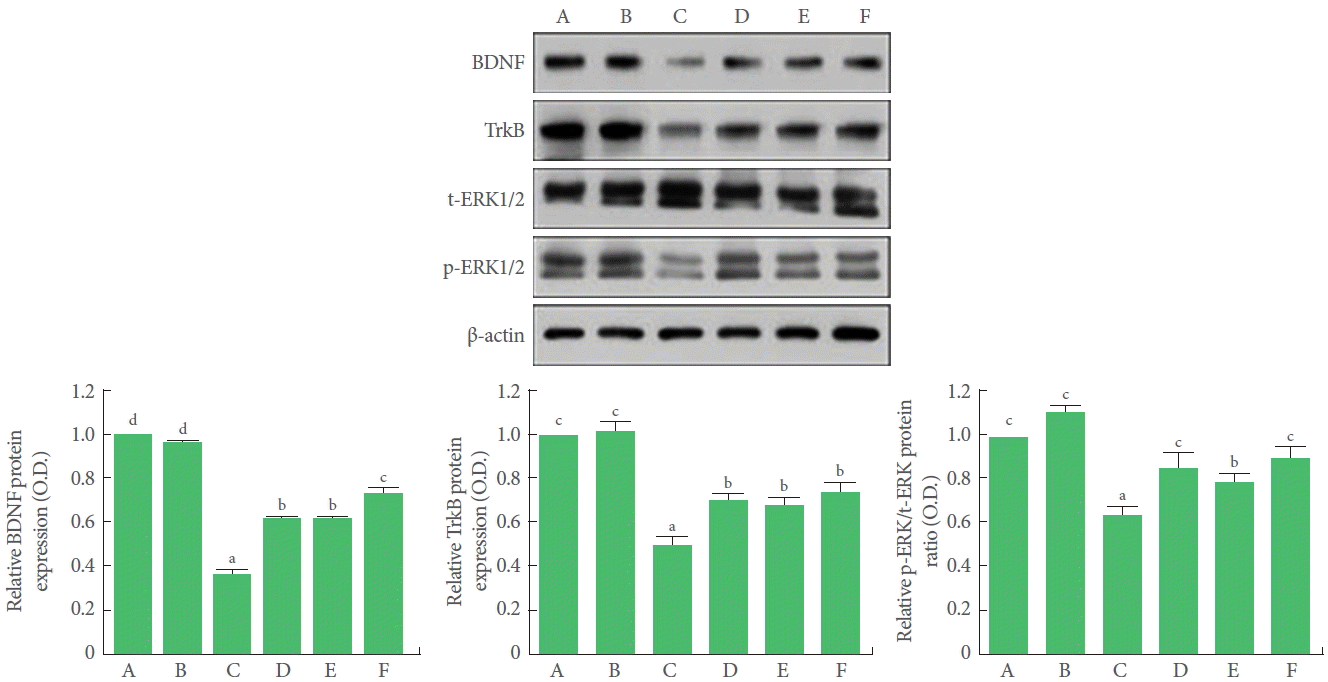
Effects of transplantation of bone marrow stromal cells (BMSCs) and treadmill exercise on the expressions of brain-derived neurotrophic factor (BDNF), tyrosine kinase B (TrkB), phospho-extracellular signal-related kinase 1 and 2 (p-ERK1/2), and total extracellular signal-related kinase 1 and 2 (t-ERK1/2). A, control group; B, treadmill exercise group; C, traumatic brain injury (TBI)-induced group; D, TBI-induced and treadmill exercise group; E, TBI-induced and BMSC transplantation group; F, TBI-induced and BMSC transplantation with treadmill exercise group. The letters a–d denote statistically significant differences, P<0.05.
Effects of BMSC Transplantation and Treadmill Exercise on Bax and Bcl-2 Expressions
Bax expression in the hippocampus was significantly increased and Bcl-2 expression in the hippocampus was significantly decreased by TBI induction. Treadmill exercise and BMSC transplantation independently decreased Bax expression in the TBI-induced rats. Treadmill exercise significantly increased Bcl-2 expression in the TBI-induced rats. Combination of BMSC transplantation with treadmill exercise did not decrease Bax expression, but significantly increased Bcl-2 expression (Fig. 6).
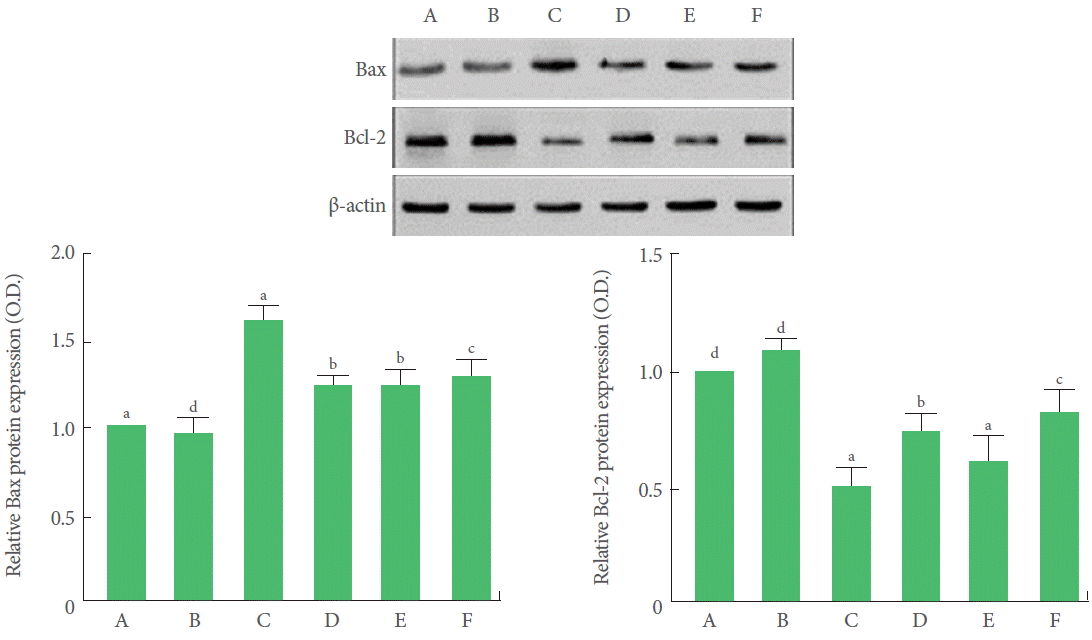
Effects of transplantation of bone marrow stromal cells (BMSCs) and treadmill exercise on Bax and Bcl-2 expressions. (A) Control group, (B) treadmill exercise group, (C) TBI-induced group, (D) TBI-induced and treadmill exercise group, (E) TBI-induced and BMSC transplantation group, (F) TBI-induced and BMSC transplantation with treadmill exercise group. The letters a–d denote statistically significant differences, P<0.05.
DISCUSSION
BMSC transplantation has been suggested as a useful strategy for cell-based therapy in stroke and TBI [21]. It promotes functional recovery by increasing endogenous neurogenesis after TBI [15]. Transplanted BMSCs were shown to survive, and improve neuronal behaviors in rats with TBI [17]. Treadmill exercise alleviated TBI-induced impairments in short-term memory and spatial learning ability by suppressing apoptosis in the hippocampus [4,20]. In this study, induction of TBI deteriorated short-term memory and spatial learning ability in rats. BMSC transplantation and treadmill exercise independently improved both short-term memory and spatial learning ability in the TBI-induced rats. We showed that transplantation of BMSCs has a stronger effect on improving spatial learning ability.
Reduction in the number of hippocampal neurons is associated with impaired performance in some hippocampus-dependent tasks [2]. Hippocampal neurogenesis is implicated in hippocampus-dependent learning ability [2,22]. The increasing effect of treadmill exercise on neurogenesis in the hippocampus has been well documented [7,18] and BMSCs are known to possess the ability to promote endogenous repair mechanisms and to replace dying neurons [23]. In the current study, induction of TBI decreased hippocampal neurogenesis, and the combination of BMSC transplantation with treadmill exercise had a strong effect on increasing neurogenesis.
Exercise increases BDNF and TrkB expression, and subsequently the BDNF-TrkB signaling pathway promotes neurogenesis and inhibits apoptosis in the hippocampus [4,6,7]. ERK1/2 is activated by BDNF-TrkB signaling, and ERK1/2 activation mediates neurotropic actions such as neurogenesis [8,24]. BMSCs act as the resource for many trophic and growth factors that are implicated in differentiation, cell survival, and angiogenesis [25,26]. Treadmill exercise protects neurons against lipopolysaccharide exposure by increasing BDNF expression in the hippocampus [19]. Treadmill exercise enhanced BDNF expression in the hippocampus, which contributes to its anti-apoptotic effect [4]. Here we show that induction of TBI inhibited BDNF and TrkB expression and inactivated the ERK pathway in the hippocampus. BMSC transplantation and treadmill exercise enhanced the expression of BDNF and TrkB, and as a result, activated the ERK1/2 pathway in the TBI-induced rats.
In our study, induction of TBI accelerated apoptosis in the hippocampus and TBI caused long-lasting cognitive impairment in rats [9]. Treadmill exercise and BMSC transplantation independently inhibited apoptosis in the TBI-induced rats. Combination of BMSC transplantation with treadmill exercise showed a stronger suppressing effect on apoptosis.
In the present study, combination of BMSC transplantation with treadmill exercise significantly increased neurogenesis and decreased apoptosis in the hippocampus. The present study shows that treadmill exercise may aid the therapeutic effect of BMSC transplantation on TBI.
Notes
Fund Support
This work was done with the sports promotion fund from Korea Institute of Sport Science, Seoul Olympic Sports Promotion Foundation (KISS-14-A05005).
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
