Current Opinion on the Role of Neurogenesis in the Therapeutic Strategies for Alzheimer Disease, Parkinson Disease, and Ischemic Stroke; Considering Neuronal Voiding Function
Article information
Abstract
Neurological diseases such as Alzheimer, Parkinson, and ischemic stroke have increased in occurrence and become important health issues throughout the world. There is currently no effective therapeutic strategy for addressing neurological deficits after the development of these major neurological disorders. In recent years, it has become accepted that adult neural stem cells located in the subventricular and subgranular zones have the ability to proliferate and differentiate in order to replace lost or damaged neural cells. There have been many limitations in the clinical application of both endogenous and exogenous neurogenesis for neurological disorders. However, many studies have investigated novel mechanisms in neurogenesis and have shown that these limitations can potentially be overcome with appropriate stimulation and various approaches. We will review concepts related to possible therapeutic strategies focused on the perspective of neurogenesis for the treatment of patients diagnosed with Alzheimer disease, Parkinson disease, and ischemic stroke based on current reports.
INTRODUCTION
As the aging population increases, common neurological disorders including Alzheimer disease (AD), Parkinson disease (PD), and ischemic stroke (IS) have increased and become important health issues with increasing socioeconomic burdens throughout the world [1].
The clinical characteristics of AD are related to progressive memory loss and cognitive deterioration. The neuropathological traits of AD are massive neuronal death with senile plaques, which are formed by the aggregation of amyloid-β (Aβ) peptides, and neurofibrillary tangles, which form from abnormal hyperphosphorylation of cytoskeletal tau protein [2,3]. These pathological changes in the brains of AD patients represent important targets for diagnosis and treatment [3].
The motor symptoms and mechanisms of PD are well known, including age-dependent uncontrollable tremors, postural imbalance, and slowness of movement and rigidity which are caused by the degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNc) located in the midbrain [4]. The neuropathological hallmarks of PD are eosinophilic intracellular inclusion bodies termed Lewy-bodies, and argyrophilic processes (Lewy neurites) [5].
The pathophysiology of IS is provoked by a reduction or complete blockage in blood supply to the brain, leading to dysfunction in the ischemic area [6]. The main causes of ischemia are thrombosis, embolism, systemic hypoperfusion, or lacunar infarction from small vessel disease. Various neurologic deficits remain after IS attacks.
There is currently no effective therapeutic strategy for addressing the neurological deficits after the development of these major neurological disorders. However, adult neurogenesis has become a topic of interest, since it was reported that the brain has the capability to generate new neurons from self-renewing and multipotent adult neural stem cells (NSCs) placed in the subventricular zone (SVZ) and subgranular zone (SGZ) of the dentate gyrus [7-11]. Therefore, the objective of this review is to evaluate possible therapeutic neurogenesis strategies for the treatment of neurological deficits in patients diagnosed with AD, PD, and IS.
BASIC CONCEPTS OF NEUROGENESIS IN THE ADULT BRAIN
Altman and colleagues first described continuous adult hippocampal neurogenesis in the rat brain in 1965, and this finding changed the idea that it was not possible for the mammalian brain to generate new neurons [12]. In addition, Temple reported multipotent, self-renewing progenitor and stem cells in the SVZ in 1989 [13]. Numerous studies have supported and reinforced these theories since that time [5,7-11,14-17]. Therefore, it is believed that adult NSCs located in the SVZ and SGZ of the dentate gyrus have the ability to proliferate and differentiate in order to replace lost or damaged neural cells throughout life. NSCs are able to differentiate into neurons and glial cells including astrocytes, oligodendrocytes and ependymal cells [18].
Diseased or damaged neurons in patients with neurological disorders lead to problems in normal function of synaptic transmission which is associated with axonal and dendritic degeneration [19]. Therefore, impaired adult neurogenesis occurs in patients with neurological diseases including AD, PD, and IS, and this leads to deterioration in the adult brain’s endogenous capacity for cell renewal in addition to loss of existing neurons due to the disease process and normal aging. However, NSCs in the adult brain can also be activated during disease processes. Previous studies have reported that stromal cell-derived factor-1α promoted neurogenesis via activation of NCSs in the adult brain during the disease process [20,21]. Exposure of stromal cell-derived factor-1α to quiescent NSCs enhances proliferation, promotes chain migration and transmigration, and activates intracellular molecular pathways mediating engagement [20]. In addition, individual neurogenesis might be affected from the perspective of the neurodevelopmental process. A recent study using human cells reported that there were deficits in the generation of hippocampal granule neurons from schizophrenic human pluripotent stem cell-derived hippocampal NCSs with reduced neuronal activity and frequency of spontaneous neurotransmitter release [22]. Therefore, determination of a clear mechanism in the development and activation of endogenous neurogenesis may be an ideal option for screening and treatment of neurological diseases. However, a therapeutic strategy for the treatment of neurologic diseases using endogenous neurogenesis is limited because of the continuous decline in the number and capacity of NCSs due to the disease process and aging [6,23]. Consequently, many studies have attempted to evaluate the efficacy of exogenous stem cell transplantation into the brains of patients with neurologic diseases [24-28]. However, unexpected complications have been reported including the inability of these cells to differentiate into specific types of neuron, and the risk of malignant transformation and immune rejection after NSCs transplantation [6].
ADULT NEUROGENESIS IN ALZHEIMER DISEASE
German psychiatrist and neuropathologist Alois Alzheimer first described the most common form of dementia in 1906. AD is the most frequent type of dementia that occurs in middle to late life [29]. It is characterized by widespread neurodegeneration throughout the basal forebrain, cortex, and limbic system as a result of neuronal and synaptic loss [30]. Neuropathologic hallmarks of AD are the presence of amyloid plaques and neurofibrillary tangles. Aβ is the product of amyloid precursor protein (APP) proteolysis by β and γ-secretase enzymes [31].
In the genetic aspect, mutations in the substrate APP and in the γ-secretase component presenilin 1 and 2 have been reported to cause familial AD [32-34]. These genetic mutations induce development of the toxic Aβ oligomers and result in deposition and accumulation of Aβ species, especially of the Aβ42 peptide in intracellular and/or extracellular spaces [30,31]. A study using triple transgenic mice harboring three mutant genes (APP, presenilin 1, and tau) showed that the reduction in neurogenesis was directly associated with the presence of Aβ plaques in the hippocampus [35]. In addition, Aβ induced alterations in GABAergic neurotransmission or an imbalance between GABAergic and glutamatergic neurotransmission, both of which contributed to impaired neurogenesis in AD [30,36]. Therefore, it is believed the production of toxic Aβ42 should be one of the important targets to enhance neurogenesis in AD patients. It is now well accepted that the increase in Aβ42 plaques is due to a reduction in the efficiency of γ-secretase to process its substrate rather than an increased production of Aβ [37,38].
Initially, many researchers investigated the clinical efficacy of γ-secretase inhibitors for AD patients to prevent the production of toxic Aβ42 peptides. However, many side effects including cognitive decline, weight loss, skin cancers and gastrointestinal infections induced by the inhibition of Notch processing were found in clinical trials [39-41]. Modulators of γ-secretase have been investigated as new drugs for AD that could preferentially increase the concentration of the shorter nontoxic Aβ species from longer toxic forms [38,42,43] (Fig. 1). However, further investigations including AD transgenic animal models under similar conditions and clinical trials are needed to prove the efficacy and safety of the γ-secretase modulators.

Stepwise cleavage processing of the β-carboxyl terminal fragment of β-amyloid precursor protein by γ-secretase generates Aβ. (A) Normal: γ-secretase cleaves cleavage site 99 sequentially and Aβ production steps forward via 2 product lines (from Aβ49 to Aβ40 or from Aβ48 to Aβ42). (B) An Alzheimer disease patient with a presenilin mutation: occurrences of inappropriate cleavage lead to an increase in the Aβ42:Aβ40 ratio and produce longer toxic forms of the Aβ peptide. (C) γ-secretase modulator: the γ-secretase modulator preferentially enhances cleavage activity leading to production of the shorter nontoxic Aβ species Aβ38 and Aβ37 from the longer toxic forms.
The apolipoprotein E4 (apoE4) allele is the major genetic risk factor for sporadic AD due to the higher prevalence and earlier onset of AD in apoE4 carriers [44]. In response to central nervous system stress or injury, neurons can synthesize ApoE to protect against neuronal injury or to promote neuronal regeneration [45]. However, ApoE4 among the ApoE family uniquely undergoes neuron-specific proteolysis, resulting in bioactive toxic fragments that enter the cytosol, alter the cytoskeleton, disrupt mitochondrial energy balance, and cause cell death [46]. Li et al. demonstrated that ApoE4 altered signaling that promoted glial differentiation leading to a detrimental effect on adult hippocampal neurogenesis [47]. Accumulating data suggest the neurodegenerative toxic effect of ApoE4 is caused by a domain interaction (Arg-61 in the N-terminal domain interacts with Glu-255 in the C-terminal domain) [44-46]. Therefore, agents capable of converting ApoE4 to an ApoE3-like molecule by disrupting the domain interaction would be one of the potential therapeutic strategies for promoting neurogenesis in AD patients [46] (Fig. 2).
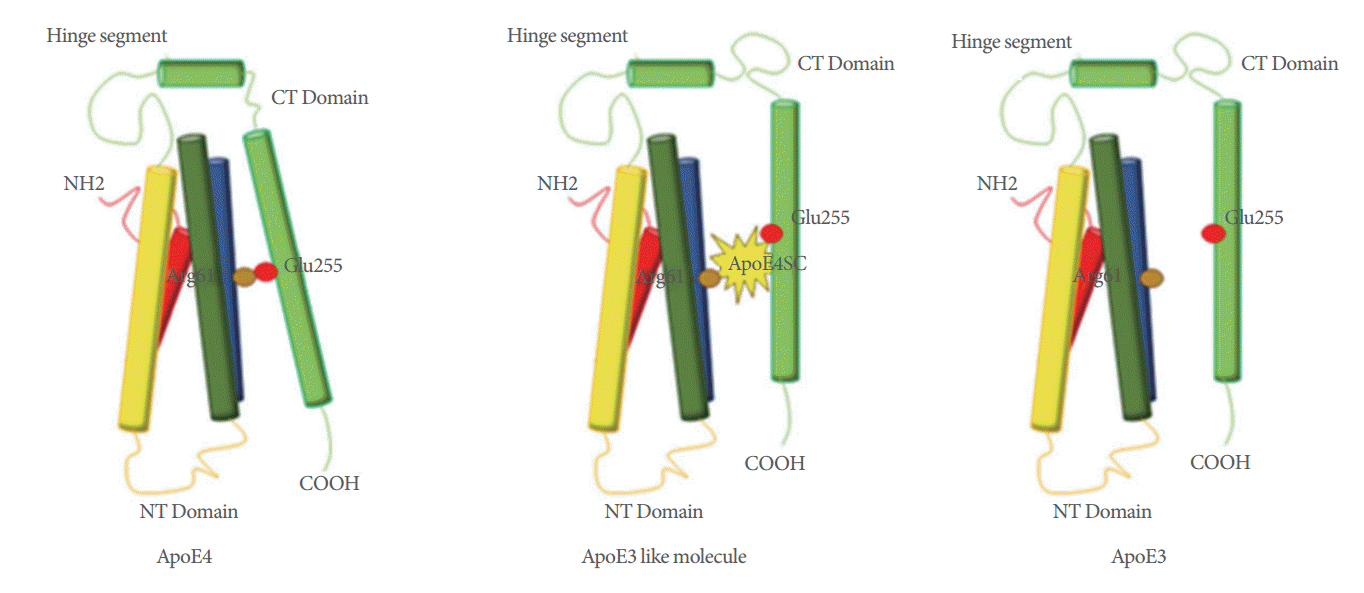
Apolipoprotein E4 (ApoE4) domain interaction is caused by the ionic interaction between arg-61 in the amino-terminal domain and glu-255 in the carboxyl-terminal domain. The ionic interaction between arg-61 and glu-255 in the ApoE4 domain can be blocked by a small-molecule which converts ApoE4 to an ApoE3-like molecule both structurally and functionally. CT, carboxyl terminus; NH, amine; NT, amino terminus; COOH, carboxyl.
Mesenchymal stem cells (MSCs) inhibit apoptosis and inflammation, modulate the immune response in damaged tissues, and promote endogenous neurogenesis and neuroprotection [48,49] Repeated administration of human umbilical cord blood MSCs into the cerebrospinal fluid of the mouse resulted in enhancement of endogenous adult hippocampal neurogenesis and synaptic activity through the paracrine actions of growth differentiation factor-15, which is a human umbilical cord blood-MSC-secreted paracrine factor, suggesting a possible role for human umbilical cord blood-MSCs as a therapeutic agent for AD [50]. In addition, NSC transplantation in APP/presenilin 1 transgenic mice significantly improved cognitive deficits and decreased the expression of proinflammatory mediators via suppression of the glial and toll-like receptor 4 (TLR4) inflammatory pathway [51]. This data suggests that these inflammatory pathways may potentially be important therapeutic targets to prevent or delay AD.
Urinary incontinence often occurs in patients suffering from AD. A recent study showed an altered voiding behavior in a transgenic mouse model of AD [52]. The authors explained that the reason of voiding alterations in the APP/presenilin 1 of mice could be because of the changes in related to anxiety and general locomotor behavior, specific of AD. The exact underlying mechanism between AD and altered voiding behavior needs to be elucidated in future research.
ADULT NEUROGENESIS IN PARKINSON DISEASE
PD is the second most common neurodegenerative disorder after AD [29]. PD is well known as a progressive, chronic neurodegenerative disease causing motor disorders such as hypokinesia, rigidity, tremor, and postural instability as well as exhibiting nonmotor symptoms including depression, anxiety, cognitive and olfactory deficits, and autonomic dysfunction [30]. The pathological hallmarks of PD, include Lewy body dementia, loss of dopaminergic neurons in the substantia nigra pars compacta and formation of Lewy bodies and Lewy neurites in surviving neurons [4]. Currently, there is no treatment to prevent disease progression and neurodegeneration, although administration of L-dopa temporarily relieves parkinsonism symptoms [4].
It is accepted that LIM homeobox transcription factor (Lmx)1a/b and Msh homeobox 1 (Msx1) which are induced by Lmx1a are critical intrinsic factors related to dopamine neurogenesis [53-56]. Multiple factors include two critical pathways (i.e., sonic hedgehog [Shh]- Forkhead box protein [Fox] A2 and Wnt1-Lmx1a) which are associated with the development of midbrain dopaminergic neurons. Nuclear receptor related 1 protein (Nurr1) is also known as a key regulator of midbrain dopaminergic neurons [57] (Fig. 3). Therefore, reactivation of these factors involved in dopamine neurogenesis during development may suggest essential therapeutic strategies for PD. Recently, Hedlund et al. [58] reported that Lmx1a and other progenitor markers remained in the midbrain aqueductal zone beyond the normal period of dopamine neurogenesis and their proliferation could be stimulated by dopamine receptor antagonists, leading to increased neurogenesis. These results indicate the potential for reactivation of dopamine neurogenesis in adult midbrain dopamine progenitor cells. In addition, Nurr1 agonists showed neuroprotective effects on midbrain dopaminergic neurons and were associated with significant improvements in behavioral deficits in a rat model of PD [57].
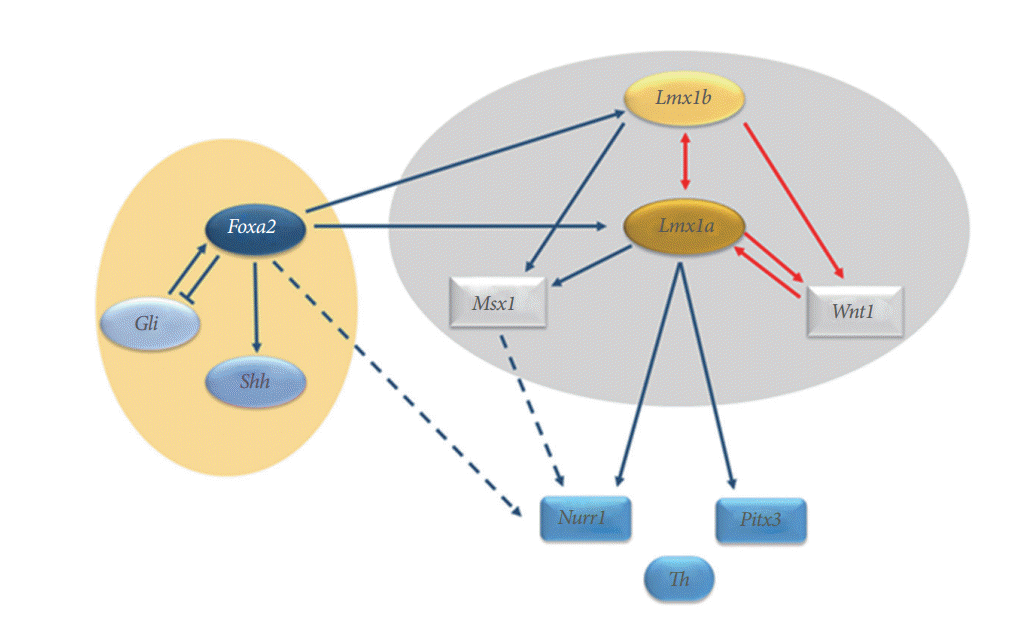
Lmx1b expression in the midbrain directly regulates the expression of Wnt1 and Lmx1a. The mutual regulation activities are shown between Lmx1b and Lmx1a and between Lmx1a and Wnt1, forming an auto-regulatory loop (red arrows). Foxa2 directly regulates Shh and Lmx1a/b to induce specification of midbrain dopaminergic neurons. Lmx1a directly regulates the expression of key regulators of midbrain dopaminergic neurons, such as Nurr1 and Pitx3, which in turn regulate tyrosine hydroxylase. The Nurr1 and Pitx3 genes are also regulated by Foxa2, Msx1, and Wnt1. Lmx, LIM homeobox transcription factor; Fox, Forkhead box protein; Shh, sonic hedgehog; Nurr1, nuclear receptor related 1 protein; Pitx3, paired like homeodomain 3; Th, tyrosine hydroxylase; Msx1, Msh homeobox 1.
α-Synuclein is a well-known modulator of adult neurogenesis and is a key protein in PD and Lewy body dementia [59]. Winner and colleagues reported that increasing amounts of α-synuclein were associated with a negative impact on adult hippocampal neurogenesis and dendritic development in newborn neurons [60]. Activation of the cAMP response element-binding protein (CREB) pathway by the phosphodiesterase inhibitor rolipram showed partial improvement of the dendrite outgrowth defect in mice overexpressing α-synuclein [60]. A recent study using a transgenic rat model of PD showed that accumulating α-synuclein and impaired 5-HT neurotransmission severely affected hippocampal neurogenesis prior to the onset of aggregation pathology and motor deficits [61].
Leucine rich repeat kinase 2 (LRRK2) is a large multidomain protein bearing GTPase and kinase activity, and mutations in this gene represent one of the stronger risk factors for the development of Parkinson disease [62,63]. Although the underlying pathogenesis of PD remains poorly understood, increased LRRK2 kinase activity, which is caused by the G2019S mutation, is thought to be associated with LRRK2-linked PD [64] (Fig. 4). Several studies have shown dopaminergic neurodegeneration from cultured dopaminergic neurons of pluripotent stem cells from PD patients harboring the LRRK2-G2019S mutation and human LRRK2-G2019S-expressing transgenic mice [65-67]. The potential relationship between LRRK2, α-synuclein, and tau in inducing PD pathogenesis has been suggested [62, 63,68,69]. LRRK2 functions upstream of pathogenic effects through α-synuclein, tau or both proteins [70] (Fig. 4). Therefore, PD pathogenesis induced by LRRK2 may be a potential new target as a therapeutic strategy for patients with PD.
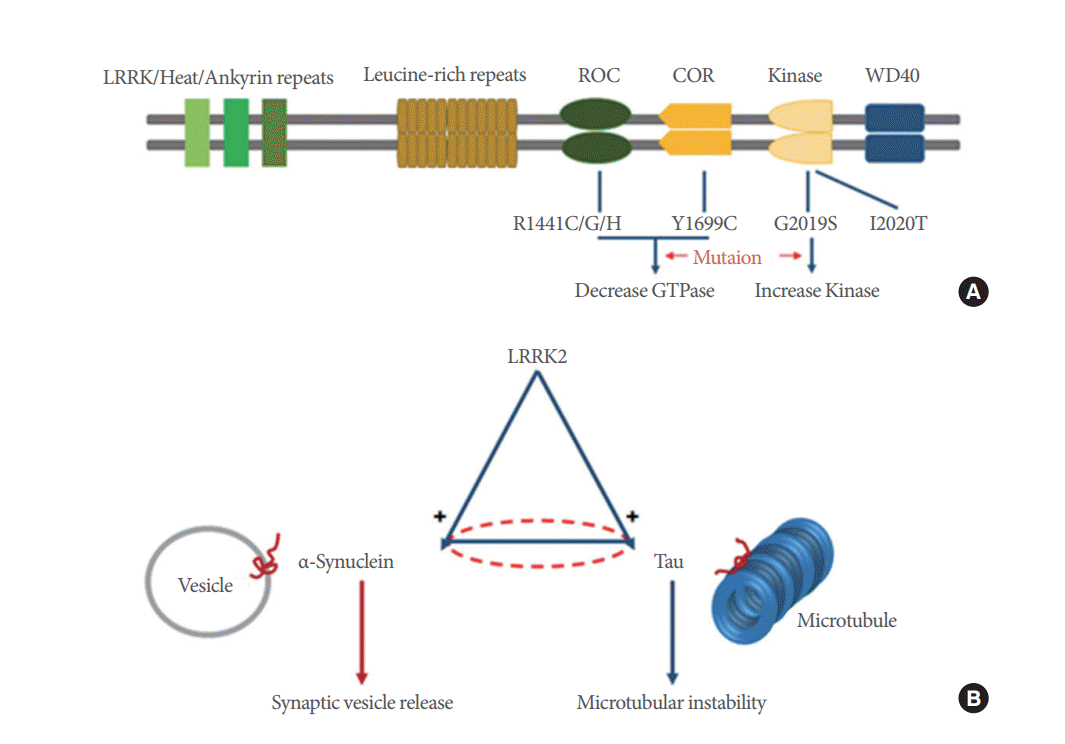
Schematic drawings of domains and mutations of leucine rich repeat kinase 2 (LRRK2) and the relationship between LRRK2, α-synuclein and tau protein. (A) LRRK2 is a large multidomain protein containing GTPase and kinase activity and mutations. LRRK2 domains are composed of a GTP-binding ras of complex protein (ROC) domain, a carboxy-terminal of ROC (COR) domain and a kinase domain. Both R1441 and Y1699 mutations in LRRK2 decrease GTPase activity, whereas G2019S increases kinase activity in LRRK2. (B) LRRK2 functions upstream of pathogenic effects through α-synuclein, tau or both proteins. The mutual influence between α-synuclein and tau is less obvious (dashed line). Dysfunctions in α-synuclein and tau cause synaptic vesicle release and microtubular instability
In recent years, induced pluripotent stem cells (iPS cells) through somatic cell reprogramming have drawn attention from researchers because of their many beneficial effects on neurodegenerative diseases such as PD [71]. In addition, iPS cells can be generated from autologous cells and are able to overcome the barriers of allogenic cell transplantation [72,73]. Han and colleagues demonstrated that PD rats with iPS cell-derived NSCs transplanted into the striatum showed improvement in functional defects of rotational asymmetry. In addition, iPS cell-derived NSCs were found to survive and integrate into the brain of transplanted PD rats and differentiate into neurons, including dopaminergic neurons in vivo [71]. Based on these findings, clinical application of iPS cells for neurodegenerative diseases, including PD, may be an important new therapeutic strategy in the near future.
It is well known that the loss of dopaminergic activity (physiologic inhibition of the micturition reflex mediated by dopaminergic D1 activity) leads to overactivity of the micturition reflex [74]. The pontine micturition center or Barrington’s nucleus is gaining particular importance due to: (1) recent findings of α-synuclein in Barrington’s nucleus, (2) known urinary dysfunction in parkinsonian patients, other patients with dementia and in very old individuals; and (3) its proximity to the pedunculopontine nucleus, a surgical target in deep brain stimulation for PD patients. Campeau et al. [75] reported that the transplantation of bone marrow derived mesenchymal stromal cells into the substantia nigra pars compacta, which improved the urodynamic pressure by 42 days, compared to the control group. The authors described that more tyrosine hydroxylase positive neurons were observed in the treated substantia nigra pars compacta.
ADULT NEUROGENESIS IN ISCHEMIC STROKE
Stroke remains a major cause of morbidity and mortality around the world [76]. There are various reasons for IS occurrence including thrombosis, embolism, systemic hypoperfusion, or venous thrombosis. When cerebral blood flow is reduced, the affected parts of the brain experience oxygen deprivation. Decreased oxygen delivery results in activation of cellular anaerobic metabolism leading to the depletion of glucose, which is the only source of energy in the brain. This ischemic cascade causes neuronal damage and ionic pump failure in the brain due to energy depletion, and ultimately leads to necrosis and apoptosis of neurons and glial cells resulting in irreversible injury to core regions with partially reversible damage in the surrounding penumbra zone [6].
The occurrence of stroke-induced compensatory endogenous neurogenesis has been demonstrated in the adult human brain [77-79]. The SVZ is well known as the main source of NSCs that are generated after a stroke which migrate toward the damaged area [80-83]. In addition, ischemia-induced neurogenesis occurs in areas that are non-neurogenic in the intact brain (e.g., the striatum and cerebral cortex) as well as in areas where new neurons normally form, such as the SVZ and SGZ [84]. Several studies found that there is increased neurogenic activity in the ischemic penumbra distant from the SVZ as well as in the neurogenic region of the lateral ventricular wall in the human brain after stroke [77,78,85]. However, the limited number and capacity of NCSs due to stroke attacks and normal aging may lead to a decrease in the number and maturation of newly generated neurons in the ischemic penumbra of the cerebral cortex. A recent study demonstrated that stroke-generated neuroblasts (DCX+ /BrdU+) were observed in the peri-infarct cortex within two weeks after IS. However, they failed to detect the same neuroblast markers in these cells and there was no differentiation to mature neurons after 4 weeks [86].
Strategies to stimulate ischemia-induced neurogenesis seem to follow these steps: (1) proliferation of NCSs, (2) survival of immature or mature neurons, (3) migration of new neuroblasts to the appropriate location, (4) differentiation of new neuroblasts to the phenotype of neurons that need to be replaced, and (5) development of functional synaptic connectivity counteracting disease symptoms [84].
Glial cell-derived neurotrophic factor (GDNF), which is a nerve growth factor, is associated with neurogenesis after stroke [87-90]. A recent study in a rat neonatal ischemic model reported that the infusion of GDNF promoted endogenous selfrepair by stimulating proliferation of glial progenitor cells derived from both the SVZ and white matter, activating their differentiation into more mature oligodendrocytes and raising the survival rate of these newly generated glial cells [90]. In addition, phosphatidylinositide 3-kinase (PI3K) is one of the wellestablished pathways affecting cell proliferation, growth, differentiation, motility, survival, and intracellular trafficking [91] (Fig. 5). The PI3K pathway is necessary for the survival of both neurons and NSCs which are essential for endogenous neurogenesis [92-95]. Activated PI3K phosphorylates Akt (protein kinase B), which is a downstream effector. Phosphorylated Akt affects mouse double minute 2 homolog (MDM2), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB), endothelial nitric oxide synthase (eNOS), mammalian target of rapamycin (mTOR), and S6 kinase and inhibits Forkhead box O (FOXO), BAD, and glycogen synthase kinase (GSK)-3β. All of these signals contribute to the protection and neurogenesis of NSCs [91].

Phosphatidylinositol 3-kinase (PI3K) pathway: Activation of class IA/B PI3Ks occurs through the stimulation of receptor tyrosine kinases, which is induced by insulin and the concomitant assembly of receptor–PI3K complexes. Activated PI3Ks catalyze the conversion of PtdIns(4,5)P2 to PtdIns(3,4,5)P3. PtdIns(3,4,5)P3 serves as a second messenger that helps to activate protein kinase B (AKT). Through phosphorylation, activated AKT affects many important downstream signals, including mouse double minute 2 homolog (MDM2), nuclear factor kappa–light-chain-enhancer of activated B cells (NF-kB), endothelial nitric oxide synthase (eNOS), mammalian target of rapamycin (mTOR), and S6 kinase, and inhibits Forkhead box O (FOXO)s, Bcl-2-associated death promoter (BAD), and glycogen synthase kinase (GSK)-3β. RTKs, receptor tyrosine kinases; Ptdlns, phosphatidylinositol; PTEN, phosphatase and tensin homolog; SGK, serine/threonine-protein kinase; S6K, ribosomal protein S6 kinase.
Transplantation of neuronal precursors derived from human embryonic stem cells has been reported to reduce infarct volume and improve behavioral outcomes after distal middle cerebral artery occlusion in rats [96-98]. Jin et al. [96] demonstrated that transplantation increased neurogenesis in the ipsilateral SVZ, but not in the contralateral SVZ or either SGZ in both young adult (3 months old) and aged (24 months old) rats with focal cerebral ischemia. These findings suggest that transplantation of NCSs for stroke treatment may be associated with changes in endogenous adaptive processes of neurogenesis. Therefore, transplantation of NCSs with stimulation of endogenous neurogenesis may be a potential therapeutic strategy for stroke recovery in the future.
Neurogenic lower urinary tract dysfunction is a major problem in patients with various neurological disorders, and may result in debilitating symptoms and serious complications, including chronic renal failure and recurrent urinary tract infections [99]. ISs are known as inducing not only bladder overactivity, but also stress urinary incontinence [100]. After IS occurrence duloxetine, a norepinephrine and serotonin reuptake inhibitor reduced bladder overactivity but failed to enhance active urethral closure mechanisms during sneezing, suggesting that disorganization of the brain network after IS might influence the effect of duloxetine on lower urinary tract function.
CONCLUSIONS
Currently, there are no effective treatments to improve clinical outcomes of common neurological disorders such as AD, PD, and IS. Therefore, many researchers have an interest in neurogenesis as a new therapeutic strategy for various neurologic diseases. We reviewed the current reliable opinions on the mechanisms and potential clinical applications related to neurogenesis for patients with AD, PD, and IS. Although there remain many limitations in the clinical application of both endogenous and exogenous neurogenesis for AD, PD, and IS patients, the accumulated data demonstrates the possibility of overcoming these limitations in the near future. Advances in the field of neurogenesis may bring a better quality of life for patients suffering from these devastating disorders.
Notes
Grant/Fund Support
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (2015R1A2A2A04004865).
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
