 |
 |


- Search
| Int Neurourol J > Volume 22(1); 2018 > Article |
|
ABSTRACT
Purpose
Methods
Results
Conclusions
NOTES
REFERENCES
Fig.┬Ā1.

Fig.┬Ā2.
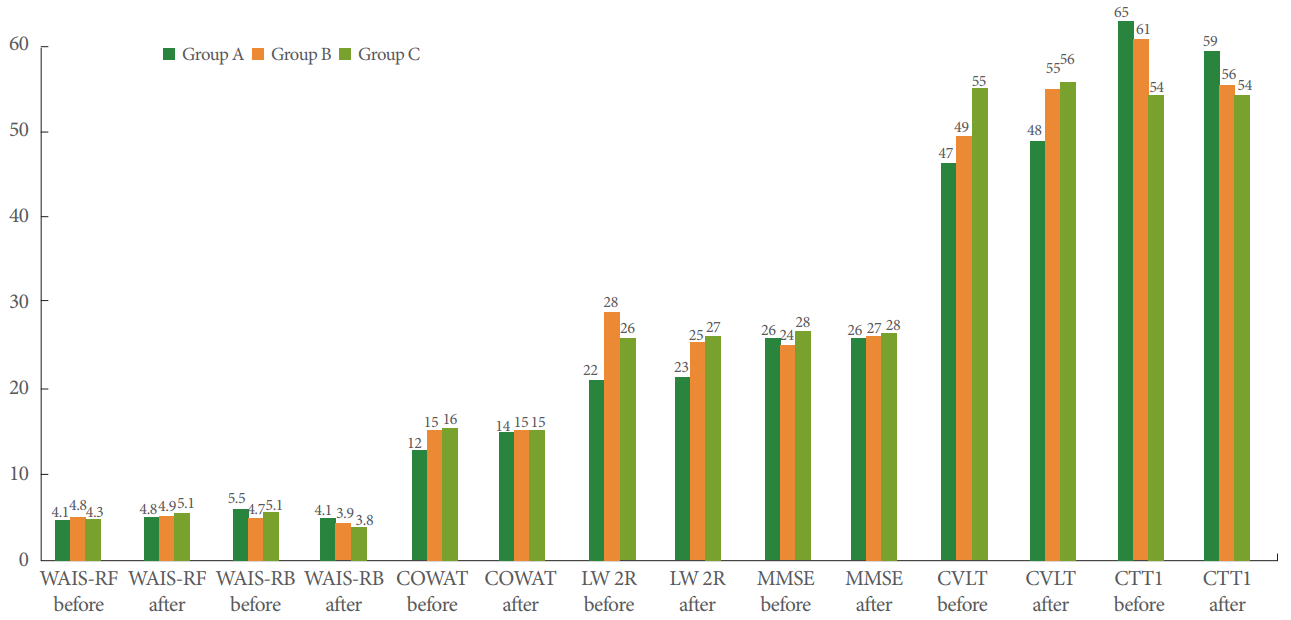
Table┬Ā1.
Values are presented as mean┬▒standard deviation.
Group A, solifenacin 20 mg + trospium 60 mg per day; group B, solifenacin 10 mg + trospium 30 mg per day; group C, control (placebo); ICIQ-SF, International Consultation on Incontinence Questionnaire-Short Form; PVR, postvoid residual (urine volume); Qaver, average flow rate; Qmax, maximum flow rate.
P-value>0.05 in all cases (differences between groups are unreliable).
Table┬Ā2.
Values are presented as mean┬▒standard deviation.
Parameter values for the patientŌĆÖs cognitive status scales and the functional state of the lower urinary tract (LUT) are given in scores, health-related quality of life (HRQoL) scales are in %, for uroflowmetry in units indicated in the table.
Group A, solifenacin 20 mg+trospium 60 mg per day; group B, solifenacin 10 mg+trospium 30 mg per day; group C, control (placebo); WAIS-R, Wechsler Adult Intelligence Scale-Revised; WAIS-RF, digit span forward; WAIS-RB, digit span backward; WMS-III, Wechsler Memory Scale III; SDFR, short-delay free recall; SDCR, short-delay cued recall; LDFR, long-delay free recall; LDCR, long-delay cued recall; MOS-36, Medical Outcomes Study 36-Item (MOS-36) Health Survey; ICIQ-SF, International Consultation on Incontinence Questionnaire-Short Form; PVR, postvoid residual (urine volume); Diary/urgency, urgency incontinenceŌĆōepisodes of day; Qaver, average flow rate.
*P<0.05, **P<0.01, comparing between the same group before and after treatment (A/A; B/B; C/C). QŌĆōmultiple comparisons with Bonferroni amendment. Critical P-value level<0.017.
Table┬Ā3.
Group A, solifenacin 20 mg + trospium 60 mg per day; group B, solifenacin 10 mg + trospium 30 mg per day; group C, control (placebo); HRQoL, health-related quality of life markers; MOS-36, Medical Outcomes Study 36-Item; PF, physical functioning; GHP, general health perceptions; PRF, physical role functioning; ERF, emotional role functioning; SRF, social role functioning; MMSE, Mini-Mental State Examination; COWAT, Controlled Oral Word Association Test; WAIS-R, Wechsler Adult Intelligence Scale-Revised; WAIS-RF, digit span forward; WAIS-RB, digit span backward; CTT1, Color Trails Test 1; CTT2, Color Trails Test 2; LM 2R, logic memory 2 recognition (WMS-III); CVLT TL, California Verbal Learning Test TL; PVR, postvoid residual (urine volume); Qaver, average volumetric rate of urination; UUI, urgency urinary incontinence; ICIQ-SF, International Consultation on Incontinence Questionnaire-Short Form (score point).
Spearmen coefficient (*P<0.05).
Table┬Ā4.
Group A, solifenacin 20 mg + trospium 60 mg per day; group B, solifenacin 10 mg + trospium 30 mg per day; group C, control (placebo); PVR, postvoid residual (urine volume); Qaver, average volumetric rate of urination; UUI, urgency urinary incontinence (episodes); ICIQ-SF, International Consultation on Incontinence Questionnaire-Short Form (score point); MMSE, Mini-Mental State Examination; COWAT, Controlled Oral Word Association Test; WAIS-R, Wechsler Adult Intelligence Scale-Revised; WAIS-RF, digit span forward; WAIS-RB, digit span backward; CTT1, Color Trails Test 1; CTT2, Color Trails Test 2; LM 2R, logic memory 2 recognition (WMS-III); CVLT TL, California Verbal Learning Test TL.
Spearmen coefficient (*P<0.05).





