Exosomes as a Communication Tool Between the Lymphatic System and Bladder Cancer
Article information
To the editor,
The late Dr. Judah Folkman (February 24, 1933–January 14, 2008), a pioneer in the field of vascular biology and medicine, was the first to propose that growing tumors need a new network of blood vessels to survive and thrive, and that inhibition of the new blood vessel growth, termed angiogenesis, could suppress tumor growth and progression [1]. Since then, numerous antiangiogenic agents and new therapies have been developed and clinically evaluated to inhibit tumor growth [2,3]. Similarly, angiogenesis of lymphatic vessels, termed lymphangiogenesis, is the process of new lymphatic vessel formation and expansion [4]. The lymphatic system is a network of lymphatic vessels that carry the interstitial fluid, cells, lipids and other large molecules, collectively called lymph. Lymph contains lymphocytes and, thus, the lymph system plays an important role in our immune system. The lymphatic system also includes secondary lymphoid tissues, such as lymph nodes, the spleen, tonsils, Peyer’s patches and mucosa-associated lymphoid tissue [4]. While the blood vascular system is a circular system, where the fluid (blood) leaves the heart and comes back to the same organ, the lymphatic system is a linear vascular system that starts as capillary lymphatic vessels, and eventually connects to the subclavian vein via the biggest caliber lymphatic vessels, called the thoracic duct. Although the blood and the lymphatic vessels share a number of similarities in their structure, anatomy, function, and more, the systems also have as many disparities in their pathophysiology.
Lymph node metastasis is considered to have a major prognostic value, boasting characteristics such as tumor aggression and a worse cancer survival rate. It is now generally accepted that lymphangiogenic factors and lymphangiogenesis promote metastasis to lymph node and distant organs [5]. Intra- and peritumoral lymphatic vessels are now hypothesized to play an important role in the interaction between lymphatic endothelial cells (LECs) and cancer cells, leading to cancer metastasis. Although the molecular and cellular mechanisms that control these processes have not yet been completely identified, the remodeling of tumor-associated lymphatics has been reported to provide important clinical information on metastasis and prognosis. In the case of metastatic melanomas with enlarged tumor-associated lymphatics, the lymphatic vessel area was significantly associated with poor disease-free and overall survival [6,7]. Thus, targeting lymphangiogenesis through inhibiting lymphangiogenic signaling pathways could be a potential tool for blocking or delaying metastasis. Both tumor cells and stromal cells secrete a number of cytokines, growth factors (such as vascular endothelial growth factors [VEGFs]), and other signaling molecules, such as exosomes, which promote lymphatic enlargement and remodeling. Therefore, cancer-associated lymphangiogenesis may well be a strong prognostic indicator for the risk of lymph node metastasis in various cancer types [8,9].
We have previously reported 3 fluorescent lymphatic reporter animals (2 mouse lines and 1 rat model). Two transgenic mouse models, Prox1-EGFP [10] and Prox1-tdTomato [11], harbor bacterial artificial chromosome (BAC) where EGFP and tdTomato reporter genes were inserted respectively under the promoter of Prox1 gene, which functions as the master regulator of lymphatic development. The fluorescent lymphatic reporter rat was generated using the mouse Prox1-EGFP BAC [12]. These reporter models have been very useful tools for the lymphatic researches, allowing direct visualization of the lymphatic networks, for example, on the surface of the bladder (Fig. 1).
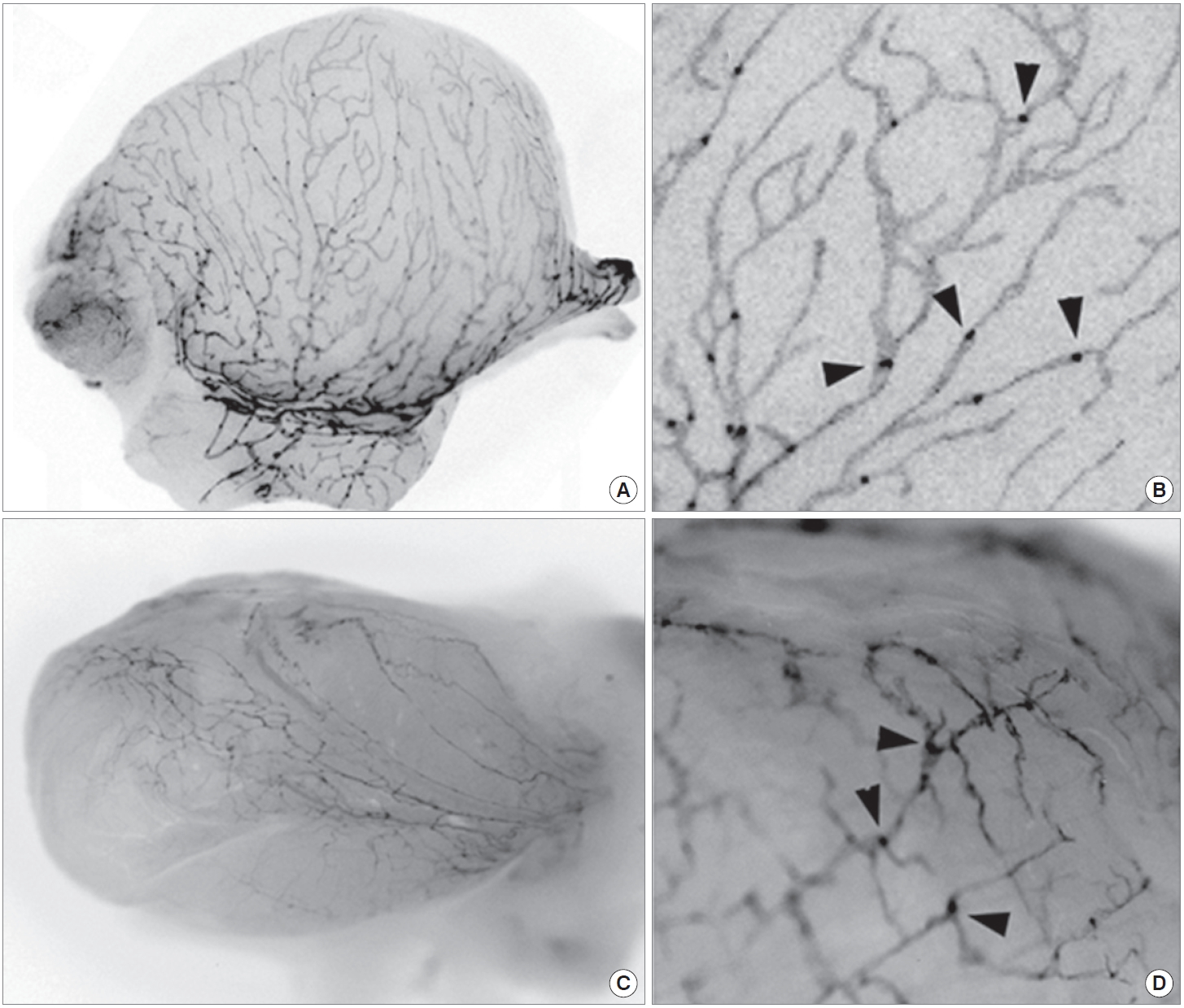
Visualization of the bladder lymphatic networks using fluorescent lymphatic reporter mouse and rat. Bladders were isolated from adult Prox1-EGFP mouse (A, B) and rat (C, D), and subjected to fluorescent stereomicroscopy. Fluorescent signals were captured using a monochrome camera and shown in grayscale. (A, C) Gross images revealed a dense network of lymphatic vessels on the surface of the bladders. (B, D) Enlarged images demonstrate lymphatic vessels and sprouts along with luminal valves (arrowheads).
Tumor-associated lymphatics drain the interstitial fluids, containing various signal molecules, cells, large molecules, and biological wastes, from tumors to the lymph nodes. Most cancer cells shed different types of microvesicles (e.g., exosomes) into the extracellular environment. These microvesicles, once considered nonfunctional biological wastes, are now considered as important carriers of genetic and epigenetic information within the tumor microenvironment [13]. Among a variety of microvesicles that fit between the size range of 5–100 nm are exosomes, which have been considered as the ideal vehicle to play a role in lymphatic transport between cancer cells and the lymphatic system [14]. These nano-sized vesicles can be isolated from multiple biological fluids, including urine, plasma, pleural effusion, saliva, and lymphatic fluids [15,16]. Tumor-derived exosomes have been reported to contain nucleic acids (e.g., DNA, mRNA, miRNA, and small noncoding RNAs), proteins, and metabolites. Since exosomes have specific membrane structures, those genetic materials can be protected from degradation in the harsh extracellular environment. Tumor-derived exosomes can activate stroma, promote cancer metastasis and chemo-resistance, and contribute to inflammatory responses, endothelial cell migration, and angiogenesis. In cancer-endothelial cell interactions, oncogenic exosomes might stimulate lymphangiogenesis and potentially enhance cancer cell invasion. Proteomics profiling of the cancer-derived exosomes showed that exosomes are enriched with proteins, whose functions are associated with cell adhesion, cytoskeletal remodeling, and signal transduction [17]. On the contrary, a recent study demonstrated that exosome-rich endothelial vesicles released from LECs accumulate in the perivascular stroma and guide the migration of dendritic cells [18]. This study provides an important implication that the LEC-secreted exosomes likely attract cancer cells and guide their metastasis.
Bladder cancer is the second most common genitourinary malignancy [19-21]. Compared to nonmuscle invasive bladder cancer (NMIBC), which is curable through surgical intervention, muscle invasive bladder cancer (MIBC) generally shows a worse prognosis [22-24]. Several recent studies suggest that lymphangiogenesis is associated with the muscle invasiveness of bladder cancers. The density of peritumoral lymphatics have been correlated with both regional metastasis and survival in bladder cancer [25,26], and upregulation of a key lymphangiogenesis factor, VEGFC, was found in bladder cancers [27]. In addition, downregulation of BLACAT2 (bladder cancer-associated transcript 2), a long noncoding RNA, is positively associated with lymphangiogenesis and invasiveness [28]. Given these findings showing that lymphangiogenesis plays an important role in bladder cancer metastasis, it is necessary to determine the roles of lymphatic systems in the development of MIBC from NMIBC. In addition, tumor-derived exosomes may have a biological function in conditioning lymphangiogenesis within the bladder microenvironment. Our own data show that exosomes isolated from T24 bladder cancer cells enhance the proliferation and migration of cultured human LECs (Fig. 2), suggesting a potential regulatory mechanism of tumor-derived exosomes in lymphatic systems through the horizontal transfer of genetic and epigenetic information.
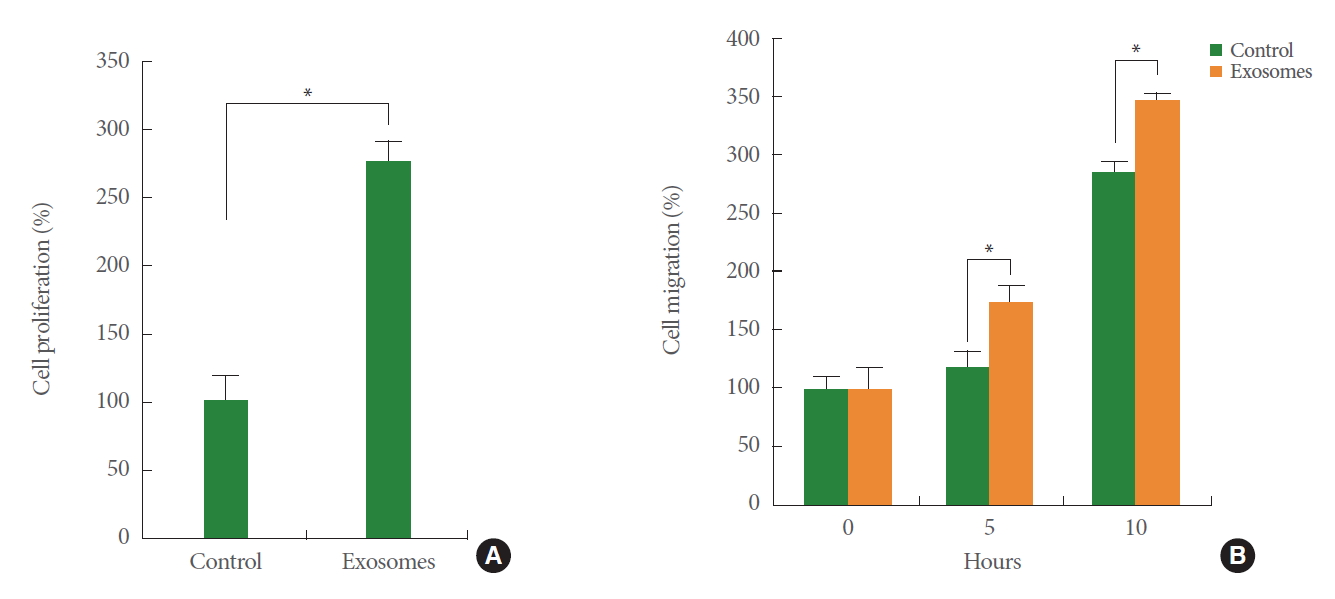
Bladder cancer cell-derived exosomes enhance the proliferation and migration of cultured lymphatic endothelial cells (LECs). Conditioned medium was harvested from cultured T24 bladder cancer cells (American Type Culture Collection, ATCC) and subjected to the ultracentrifugation process to isolate tumor-secreted exosomes. Human dermal LECs (ATCC) were treated with the isolated exosomes or phosphate buffered saline (PBS) (control). Migration (A) and proliferation (B) was compared between PBS-treated LECs and exosome-treated LECs. *P<0.05.
Despite, the casual link between exosomes and lymphatic metastasis of bladder cancer remains to be firmly established. For example, what specific bioactive materials are encapsulated or enriched in the tumor-derived exosomes that stimulate the lymphatics? And what signaling cascades are activated by exosome exposure in LECs? More studies are needed to advance our knowledge of the impact of the bladder lymphatics in metastatic bladder cancers and their exosomes, and of the identity and nature of tumor exosome contents. Similar questions could be also asked to lower urinary tract symptoms (LUTS) including urinary incontinence, overactive bladder, interstitial cystitis/bladder pain syndrome, and urinary tract infections et al. However, not much efforts have been invested to understand the casual connection between bladder lymphatic system and LUTS [29]. Thus, our collaborative research team has started working on the biological roles of urinary exosomes in LUTS and its underlying mechanism in bladder pathogenesis. We believe that profiling exosomes using multiomics approaches may shed much-needed light to these questions, and help us better understand the underlying mechanisms and biological link between exosomes and lymphatics in bladder diseases including bladder cancer as well as LUTS.
Notes
Grant/Fund Support
This study was supported in part by National Institutes of Health (to YK Hong, R01DK114645).
Research Ethics
The animal studies were approved by Institutional Animal Care and Use Committee (IACUC), University of Southern California (PI: YK Hong).
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
