Urethral Pressure Measurement as a Tool for the Urodynamic Diagnosis of Detrusor Sphincter Dyssynergia
Article information
Abstract
Purpose
To describe a technique for urodynamic diagnosis of detrusor sphincter dyssynergia (DSD) using urethral pressure measurements and examine potential associations between urethral pressure and bladder physiology among patients with DSD.
Methods
Multiple sclerosis (MS) and spinal cord injured (SCI) patients with known DSD diagnosed on videourodynamics (via electromyography or voiding cystourethrography) were retrospectively identified. Data from SCI and MS patients with detrusor overactivity (DO) without DSD were abstracted as control group. Urodynamics tracings were reviewed and urethral pressure DSD was defined based on comparison of DSD and control groups.
Results
Seventy-two patients with DSD were identified. Sixty-two (86%) had >20 cm H2O urethral pressure amplitude during detrusor contraction. By comparison, 5 of 23 (22%) of control group had amplitude of >20 cm H2O during episode of DO. Mean duration of urethral pressure DSD episode was 66 seconds (range, 10–500 seconds) and mean urethral pressure amplitude was 73 cm H2O (range, 1–256 cm H2O). Longer (>30 seconds) DSD episodes were significantly associated with male sex (81% vs. 50%, P=0.013) and higher bladder capacity (389 mL vs. 219 mL, P=0.0004). Urethral pressure amplitude measurements during DSD were not associated with significant urodynamic variables or neurologic pathology.
Conclusions
Urethral pressure amplitude of >20 cm H2O during detrusor contraction occurred in 86% of patients with known DSD. Longer DSD episodes were associated with larger bladder capacity. Further studies exploring the relationship between urethral pressure measurements and bladder physiology could phenotype DSD as a measurable variable rather than a categorical observation.
INTRODUCTION
Detrusor sphincter dyssynergia (DSD) is defined as the loss of coordination between detrusor and external sphincter in the presence of neurologic pathology. This can occur in both the settings of neurogenic overactive bladder (e.g., spinal cord injury [SCI]) or volitional voiding (e.g., multiple sclerosis [MS]). The umbrella under which DSD falls encompasses a broad spectrum of individuals that have different underlying neurologic pathologies and impact from the condition. Over the past 40 years, there have been a few attempts to characterize DSD by electromyographic (EMG) findings. In 1981, Blaivas et al. [1] deconstructed DSD into 3 types. Type 1 was characterized by a crescendo increase in EMG activity, type 2 by clonic sphincter contractions throughout the detrusor contraction, and type 3 by sustained activity increase. Twenty years later, Weld et al. [2] conceptualized DSD as either continuous or intermittent, also based on EMG patterns. Although intriguing in concept, neither of these classification strata demonstrated sustained prognostic value when translated to the clinical setting and thus are not commonly utilized in treatment algorithms for managing patients with DSD. As a result, DSD continues to be characterized as a discrete finding, either present or absent, in neurogenic bladder populations.
Diagnosis of DSD is made through urodynamic testing. EMG and voiding cystourethrography (VCUG) are commonly used to make the diagnosis. Recent literature has shown that the 2 modalities poorly correlate and that the most sensitive diagnostic criteria may require utilizing the combination of the modalities [3-5]. Both of these modalities have limitations when used to diagnose DSD. EMG accuracy can be limited by body habitus, electrode displacement, and vary with type of electrode used. VCUG requires extra equipment, radiation exposure to the patient, and increased time and costs of the investigation.
Urethral sphincter pressure measurements during urodynamics may offer an alternative or supplemental method for diagnosing DSD and yield measurement data that can be used to better characterize physiology [6]. Currently, there are few published techniques for using urethral pressure measurements to categorize DSD and these reports have yielded inconsistent data [7,8]. Consequently, using urethral pressures to diagnose DSD is currently considered experimental by the International Continence Society (ICS) [6].
We sought to investigate the utility of using urethral pressure measurements during urodynamics to diagnose DSD in neurogenic bladder patients and to categorize patients concomitant with bladder physiology. We hypothesized that urethral pressures could be used as a third potential modality in diagnosing DSD and that DSD could be subcategorized based on differences in amplitude and duration of urethral pressure during a DSD episode.
MATERIALS AND METHODS
We abstracted data (2008–2015) from an Institutional Review Board-approved institutional database of neurogenic bladder patients referred for urologic evaluation. All patients from the database with a diagnosis of SCI or MS and urodynamic evidence of DSD based on urodynamic EMG or VCUG were included. Data from SCI and MS patients with detrusor overactivity without the presence of DSD were abstracted as a control group to compare baseline changes in urethral pressures. All urodynamic tracings and fluoroscopy images were rereviewed to confirm DSD. We excluded patients with history of prior reconstructive bladder or urethral surgery and patients whose urodynamics tracing were not available for review. Abstracted data included demographics, medical and surgical history, current medications, and urodynamic results including fluoroscopic images.
Urodynamics were performed utilizing a 7 French T-Doc (Wilmington, DE, USA) air-charged dual sensor urethral catheter with sensors located at the tip of the catheter and 6 cm proximal from the tip. The catheter was inserted per urethra until both sensors were in the bladder. The catheter was then slowly pulled back through the urethra and the urethral pressures were monitored on the more proximal sensor (furthest from tip). The external sphincter was located by noting a significant increase in urethral pressure during withdrawal and the catheter was secured with tape at the urethral meatus, which prevented further sensor migration. The study was then performed per good urodynamic principles using medium-fill cystometry (30 mL/min) and contrast infusion [9]. Continuous urethral pressures were monitored during the urodynamic study in conjunction with vesical pressures. A 10F rectal catheter was used to measure abdominal pressures when appropriate. EMG patches were placed at the 3 and 9 o’clock positions adjacent to the anus with a third electrode placed on the adductor tendon or on the bony prominence of the hip. Fluoroscopy was used to capture images of DSD during suspected episodes.
DSD was defined on VCUG if there was evidence of dilation of the bladder neck to the level of the external sphincter on fluoroscopy that occurred with detrusor contraction or on EMG if pelvic floor EMG activity increased during detrusor contraction in the absence of voluntary, valsalva or credé maneuvers [3]. We determined a DSD urethral pressure cutoff by comparing urodynamic urethral pressure measurements to a control group of SCI and MS patients without DSD. Duration of DSD episode was defined as the length of time (in seconds) of the episode of urethral pressure displacement from baseline from the moment of initial increase to the moment at which the urethral pressure stopped showing variability and returned to a consistent baseline for at least 10 seconds afterwards. Detrusor overactivity was defined as any involuntary sustained detrusor pressure rise >5 cm H2O in the absence of a rise in abdominal pressure on urodynamics, and bladder compliance was defined as abnormal if less than 15 mL/cm H2O [10]. See Fig. 1 for an example.
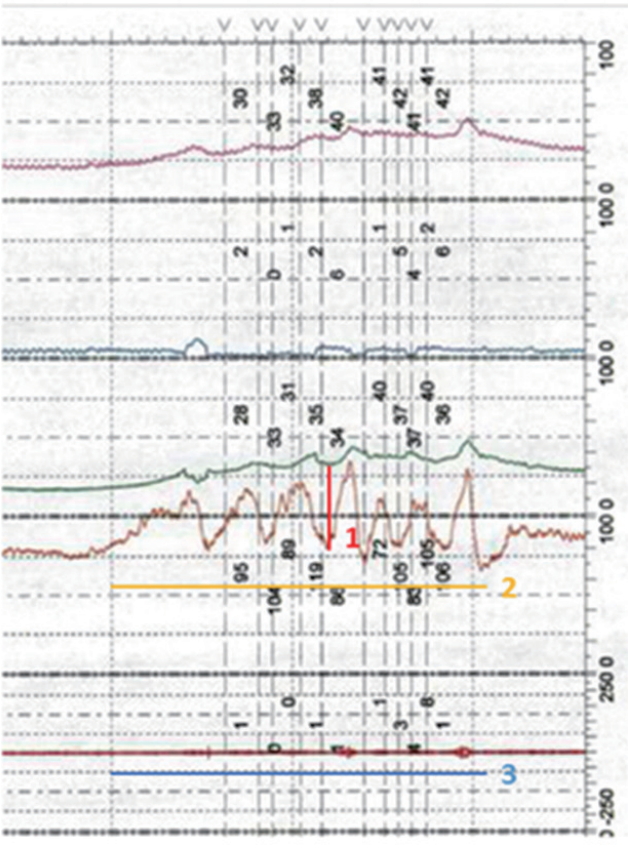
Example of urethral pressure variables and measurement. Urethral pressure amplitude as depicted by the red line (number 1) is defined as the absolute value (in cm H2O) of the maximum displacement from the baseline urethral pressure. It was defined as detrusor sphincter dyssynergia (DSD) if > or equal to 20 cm H2O. Urethral pressure duration is depicted by the orange line (number 2) and is defined as the length of time (in seconds) of the episode of urethral pressure displacement from baseline from the moment of initial increase to the moment at which the urethral pressure stopped showing variability and returned to a consistent baseline for at least 10 seconds afterwards. The blue line (number 3) highlights the electromyography (EMG) tracing. It shows an absence of EMG activity during this episode. That is to say this urodynamics tracing would be categorized as DSD based off urethral pressure, but not EMG.
We performed all statistical analyses using SAS ver. 9.4 (SAS Institute Inc., Cary, NC, USA). We compared the demographics, disease pathology, bladder physiologic parameters, as well as urodynamic findings against urethral pressure amplitude and duration.
RESULTS
We identified 128 patients with DSD based on EMG or fluoroscopic appearance with known neurological diagnosis of MS or SCI. Fifty-six were excluded having had previous surgery or lack of identifiable demographic or urodynamic data, leaving 72 individuals for analysis. Rectal pressure was not measured in all patients. Of the 58 patients who received rectal pressure measurement, 46 had EMG findings of DSD (79%). Of the 72 patients with DSD, 45 had VCUG evidence of DSD (63%), and 31 (43%) had both findings. Sixty-two of the patients (86%) had urethral pressure amplitude >20 cm H2O during either the EMG or VCUG DSD finding. The DSD episodes associated with detrusor overactivity occurred both early and late in the filling phase and the length of the episode did not vary by when DSD occurred. In the control group of randomly chosen neurogenic bladder patients with DO and no DSD on VCUG or EMG, only 5 out of 23 patients (22%) had a urethral pressure amplitude greater than 20 cm H2O during a DO episode. Of these 5, 2 of 23 (8.7%) had a urethral pressure amplitude greater than 20 cm H2O and no clear evidence of DSD whereas 3 of 23 (13%) had pressures greater than 20 cm H2O but could be interpreted as a questionable DSD diagnosis when rereviewing the EMG tracing. The distribution of urethral pressure amplitude for the cohort group during DSD episode and for the control group are depicted in Fig. 2. Based on the overall distribution of urethral pressures, 20 cm H2O was chosen as a cutoff point for DSD in this analysis, recognizing that a larger sample size is needed for a more exact discrimination threshold with a receiver operating characteristic (ROC) curve.

Urethral pressure amplitude in patients with detrusor sphincter dyssynergia (DSD) and multiple sclerosis (MS) (blue square) or spinal cord injury (SCI) (orange circle), and control patients with no known DSD (yellow triangle), and no known DSD, but with questionable DSD upon rereview of the urodynamics tracing (light blue asterisk). The green line depicts the 20 cm H2O cutoff point that was chosen to indicate urethral pressure DSD.
Demographics are presented in Table 1. The DSD cohort was predominantly male (58%) with an average age of 42 years (range, 17–79 years). 78% had SCI and 22% MS. A total of 56% of the patients had post void residual greater than 100 mL, 88% had detrusor overactivity, 68% had a bladder capacity less than 300 mL, and 28% had abnormal compliance. Average urethral pressure amplitude during DSD episode was 73 cm H2O (median, 133 cm H2O; range, 1–256 cm H2O). Average duration of episode was 66 seconds (median, 30 seconds; range, 10–500 seconds). Of the 23 control patients, 13 (56%) were female and 10 (43%) male with an average age of 45 years (range, 29–66 years). Twelve (52%) of the control group had MS whereas 11 (48%) had SCI.
When the duration of urethral pressure DSD episode was more closely examined, there were some differences in components of bladder physiology based on length of episode. Table 2 compares bladder physiologic parameters and demographics between those with shorter (≤30 seconds) and longer (>30 seconds) duration of urethral pressure DSD. Longer duration was associated with male sex (P=0.013) and larger bladder capacity (388.7 mL vs. 218.8 mL, P<0.001). There were no statistically significant differences observed between length of episode and neurologic disease, compliance, presence of detrusor overactivity, or PVR.
Multivariate analysis was performed utilizing sex, PVR, detrusor overactivity, bladder capacity, and compliance as variables When adjusted for gender, postvoid residual, presence of detrusor overactivity, bladder capacity, and compliance, the odds ratio for a longer episode (>30 seconds) of urethral pressure DSD was independently associated with male sex and increased bladder capacity (Table 3). There were no significant associations between urethral pressure amplitude and urodynamic findings.
DISCUSSION
Here we describe a technique for using urethral pressures during urodynamics for identifying patients with DSD. Diagnosing DSD can be challenging as the current methods for doing so are imperfect. EMG is limited by body habitus, electrode displacement, and is variable depending on type of electrode used. VCUG exposes the patient to harmful radiation, and increases time and costs of the study. Our study demonstrates that EMG alone diagnosed DSD 79% of the time and VCUG alone 63% of the time. In contrast, urethral pressure amplitude >20 cm H2O occurred in 86% of our sample of known DSD patients. The 20 cm H2O cutoff is relatively specific to DSD patients since only 8.7% (2 of 23) of control neurogenic bladder patients with detrusor overactivity without DSD had a urethral pressure amplitude >20 cm H2O during detrusor contraction.
The urethral pressure profile was first described by Brown and Wickham [11] in 1969 to study the effect of electrical stimulation to the pelvic floor on the closure forces of the urethra. Urethral pressure is defined as the fluid pressure needed to just open a closed urethra [12]. These definitions and measurement techniques have been standardized by the ICS [6]. Yalla et al. [7] first showed demonstrable urodynamic urethral pressure patterns in DSD in 1981. In 2001, Sakakibara et al. [13] looked at the reduction in urethral sphincter pressure during voiding for patients with DSD and found that urethral pressure decreased by an average of 6.4 and 5.0 cm H2O for men and women, respectively with DSD compared to 39 and 53 for those without [13]. Taking this information, Suzuki et al. [8] examined the utility of urethral pressure as an alternative to combined EMG/VCUG for measuring DSD. They defined urethral pressure DSD as any increase, maintenance, or decrease <10 cm H2O of urethral pressure during the voiding phase. In a 72 patient analysis, they found that only 14% of those with combined EMG/VCUG findings of DSD had DSD defined by urethral pressure. Given the discordance to what they had expected, they hypothesized that the urine flow between the wall of the urethra and the transducer could have caused a decrease of the urethral pressure. Indeed, this hypothesis is plausible, and in our study, in contrast to measuring urethral pressure during voiding alone, we further examined the urethral pressure amplitude in association with a detrusor contraction. Here one would expect to see less artifact of urine flow artificially lowering pressures, there is less risk of Valsalva voiding or changes in positioning during attempts to void. Our technique is therefore a variation from what has been previously reported in the literature and provides a new avenue for exploring the utility of urethral pressure profilometry in DSD.
Using urethral pressure measurements, we explored the possible relationship between urethral pressure data and bladder physiology. Our analysis shows that DSD patients with longer duration of urethral pressure DSD have larger bladder capacities on multivariate analysis. There were no significant associations with neurologic disease, presence of DO, or compliance on univariate or multivariate analyses. With a larger dataset, there is the potential for using urethral pressure profilimetry to better stratify risk in a population where true risk is not well defined. There have been prior attempts to correlate bladder physiology with DSD urodynamic findings, with varying degrees of success. Blaivas showed that urologic complications were not correlated with dyssynergia variable, but in stark contrast, Weld and colleagues showed a higher risk of loss of bladder compliance and renal function with a continuous DSD phenotype [1,2].
Using this small retrospective cohort, it is impossible to extensively comment on prognostic information from the duration or amplitude of urethral pressure DSD. In addition, more evidence is needed to know where urethral pressure fits in to the armamentarium of urodynamic testing. Urodynamics are utilized in an attempt to answer a wide range of clinical questions. Although urethral pressure poses an interesting potential for identifying DSD, more evidence is needed to verify how it could replace or add to the modalities that are already used in urodynamic testing. We do demonstrate how urethral pressure can be used to identify DSD in patients with known DSD and show that length of contraction may hold promise for differentiating bladder physiology among people with DSD. Further studies should explore using this as a continuous measurement in attempt to phenotype DSD. Examining DSD as a continuous numeric measure, rather than a categorical observation may yield better understanding of differences within the condition and thus better guide therapeutic decision making.
There were no detectable differences in urethral pressure amplitude or duration in those with SCI when compared to those with MS. We interpreted this to mean that the pathology of DSD does not depend on its underlying cause and DSD likely remains fundamentally the same regardless of its triggering pathophysiology. More studies will be needed to verify this.
There are limitations to consider with this study. Urethral pressure measurement is a technique that requires education and practice to ensure that external sphincter pressures are being properly recorded. It is possible that there is some variability in measurements, but the technique is utilized in all of our 800+ urodynamics studies/year, which minimalized variance in technique. An additional limitation is that the study does not account for variances in urethral pressures in the same person over time. However, the same limitation can be applied to many different urodynamic variables including compliance, capacity, and voiding pressures. A further limitation was that we were not able to subcategorize the cohort into level of injury (SCI) or type of MS due to sample size. Larger, single diagnosis cohorts will be needed to better understand urethral pressure variances within a disease type. Finally, our cutoff value of 20 cm H2O for DSD was not an exact discrimination threshold determined with an ROC curve. With a larger sample size, a calculated predictive value would strengthen conclusions drawn from the data.
In conclusion, this study describes a reproducible technique for diagnosing and categorizing DSD on urodynamic studies, in neurogenic bladder patients using a 20 cm H2O pressure change cutoff. Urethral pressure has the potential to be used as a third modality in addition to EMG and VCUG for diagnosing DSD. Episodes of DSD lasting longer than 30 seconds were associated with larger bladder capacity. With more information, urethral pressure has the potential to be used to subcategorize DSD based on differences in amplitude and duration as a measurable variable rather than a categorical observation.
Notes
Research Ethics
This study was approved by the Institutional Review Board (HUM00031859). Informed consent was obtained by all patients to be included in this study.
Conflict of Interest
No potential Conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTION STATEMENT
·Full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis: LC
·Study concept and design: LC, AC, J. QC, YQ, JS
·Acquisition of data: LC
·Analysis and interpretation of data: LC, YQ, JS
·Drafting of the manuscript: LC, JS
·Critical revision of the manuscript for important intellectual content: LC, AC, JQC, YQ, JS
·Statistical analysis: LC, YQ
Acknowledgements
The urethral pressure measurements described in this manuscript are based on thoughts developed by Edward J. McGuire, MD while at the University of Michigan.



