A Novel Technique of Morcellation Using a Pneumovesicum After Holmium Laser Enucleation of the Prostate in Complicated Situations: Our Initial Experience and Tips
Article information
Abstract
Purpose
To describe our initial experience with a novel method of adenoma retrieval using a pneumovesicum (PNV) after Holmium laser enucleation of the prostate (HoLEP).
Methods
From January 2016 to April 2018, a total of 93 consecutive patients treated with HoLEP were enrolled in this study. For tissue morcellation, we used the PNV morcellation technique for an initial series of 21 patients and the conventional technique (Lumenis VersaCut) for a consecutive series of 72 patients. We compared efficiency and safety between the novel technique and the traditional technique. Subgroup analysis was performed to assess the effectiveness of the current technique in the large prostate (>70 mL).
Results
There were significant differences in mean age and prostate volume between the 2 groups. However, there were no significant differences in the baseline characteristics and preoperative parameters in the subgroup analysis of large prostates (>70 mL). The mean morcellation efficiency was higher (8.50±1.94 minutes vs. 1.76±0.45 minutes, P<0.05) and the time of morcellation (7.81±1.25 minutes vs. 34.04±11.14 minutes, P<0.05) was shorter in the PNV group. Moreover, there were no significant differences between groups in hospitalization period (2.62±1.10 days vs. 2.90±1.26 days, P=0.852) and any other postoperative events, including recatheterization, reoperation, clot retention, and urethral stricture (P-value range, 0.194–0.447). In the PNV group, there were some cases of procedure-related complications, including postoperative extravesical leakage (5th case), clot retention (8th case), and recatheterization (9th case).
Conclusions
This method has a higher tissue retrieval efficacy, with the advantage of excellent visibility compared to conventional morcellation. The current method can be applied when a transurethral morcellator is out of order or cannot be used.
INTRODUCTION
Since its introduction by Gilling et al. [1], Holmium laser enucleation of the prostate (HoLEP) has emerged as a useful treatment option for treating bladder outlet obstruction due to benign prostatic hypelasia. The HoLEP guarantees similar and durable functional results, significantly reduced transfusion rate, shorter catheterization period, and shorter hospital stay compared to open simple prostatectomy [2,3]. Transurethral intravesical morcellation of resected prostatic adenoma is currently the standard procedure for tissue retrieval after HoLEP.
However, for many surgeons, intravesical morcellation is a very stressful procedure and serves as a barrier to mastering the HoLEP learning curve. This is because the morcellation can damage the bladder when post enucleation bleeding disturbs the vision and when the bladder is not sufficiently distended. Bladder injuries occurring during the HoLEP have been reported ranging from 1.4% to as much as 5.7% [4,5]. Regarding the site of occurrence of bladder injury, the adenoma is aspirated when the blades are directed downward towards the urinary bladder, which can cause trigonal injury or injury to the posterior wall [6]. Trigonal or posterior wall injury has a potential detrimental effect on micturition because, the trigone and bladder base have many sensory fibers [7]. A recent report also stated that trigonal injury by a morcellator resulted in a vesico-sigmoidal fistula [8].
A significant amount of research has been conducted to increase the efficacy and safety of morcellation. In particular, in a recent randomized clinical trial comparing the morcellation of Piranha (Richard Wolf, Knittlingen, Germany) and VersaCut (Lumenis Ltd., Yokneam, Israel) by Marawan, Piranha showed a slightly higher morcellation rate [9]. However, because the nephroscope lens in the morcellator is small, it is possible that some cases of poor visual acuity may occur when performing the procedure in a large prostate. Additionally, if the morcellator fails, the operation time may be delayed, or the operation may not be performed completely. Therefore, we devised a novel alternative tissue retrieval method to ensure better visibility in cases where the inevitable use of the morcellator is not possible.
MATERIALS AND METHODS
Study Design and Study Participants
After approval from the Institutional Review Board (2018-AS0132), patients who underwent HoLEP between January 2016 and April 2018 at the Korea University Ansan Hospital were enrolled in this study. One surgeon (JHB) performed HoLEP with the novel tissue retrieval technique and the traditional urethral morcellation. Patients with a history of genitourinary surgery or radiation, interstitial cystitis, genitourinary malignancy, or neurogenic bladder or who missed the preoperative transrectal ultrasonography (TRUS) evaluation were excluded from the study. Patients’ characteristics and their preoperative, intraoperative, and postoperative findings were investigated retrospectively.
The preoperative work-up comprised a medical history, physical examination, and routine laboratory tests. In addition, all the patients were evaluated preoperatively through serum prostate-specific antigen estimation, TRUS evaluation, digital rectal examination, urinalysis, and International Prostate Symptom Score (IPSS) estimation. Uroflowmetry and postvoid residual urine measurement by ultrasound were performed both preoperatively and postoperatively. Perioperatively, the enucleation and the morcellation times were recorded, and the weight of the retrieved tissue was measured.
Procedure
A detailed enucleation procedure was performed as described by Kim et al. [10]. The enucleation procedure was accomplished using a 60- to 100-W Holmium laser (Versapulse, Lumenis Ltd.) source configured with a 360-μ end-fire laser fiber. Additionally, a 26F resectoscope (Karl Storz, El Segundo, CA, USA) with a laser bridge was used. The enucleation required laser settings of 2 to 2.4 J at 25 to 50 Hz.
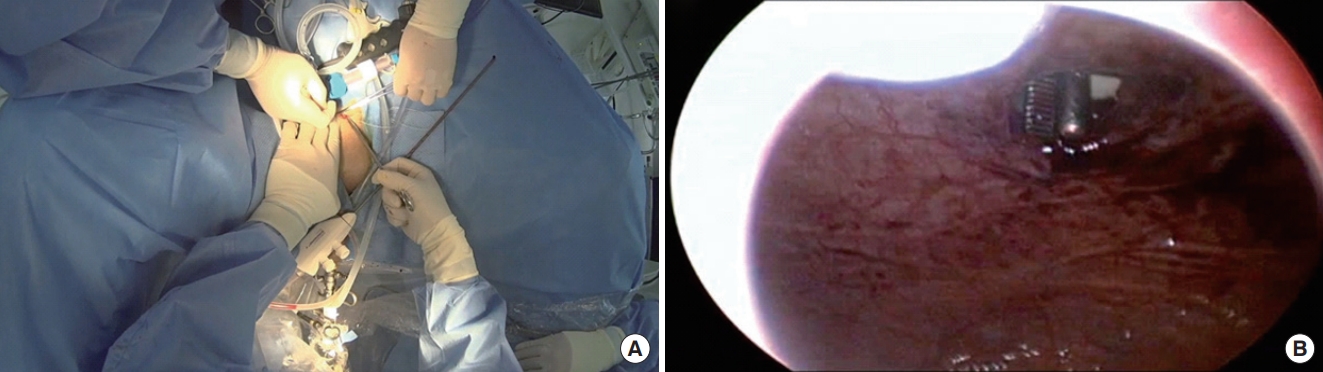
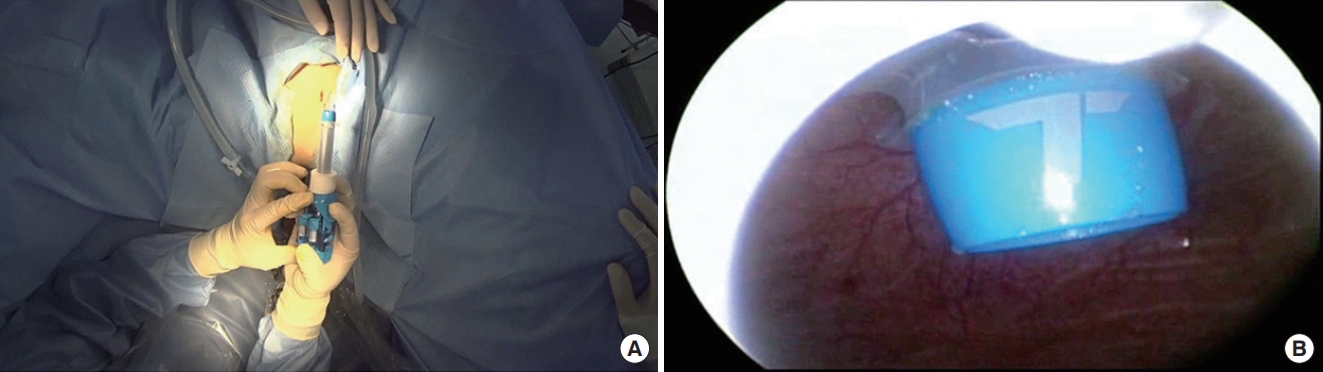
Conventional morcellation was performed under direct visualization using either the reciprocating or the oscillating morcellator (VersaCut Morcellator, Lumenis) introduced through a 26F outer sheath and the offset nephroscope (Karl Storz), with the blades facing toward the anterior bladder dome during morcellation. Otherwise, the novel technique of morcellation using pneumovesicum (PNV) is as follows. After the enucleation of the prostate adenoma, the bladder was filled with normal saline (or CO2 gas) under cystoscopy. An 11-mm laparoscopic self-retaining trocar (Transport, darim SurgNET Ltd., Seoul, Korea) was then inserted 2 cm away from the pubic symphysis (Fig. 1) under cystoscopic guidance (Fig. 2). After port insertion, the bladder was inflated with CO2 while draining the saline. Insufflation of the bladder occurred via the laparoscopic port with CO2 at a pressure of 10–12 mmHg and flow of 2–3 L/min. After the establishment of the PNV, an 11-mm electromechanical morcellator (Xcise, LiNA Medical, Glostrup, Denmark) which is commonly used in gynecological surgery, was inserted through the port under cystoscopic vision (Fig. 3). Subsequently, a toothed grasping instrument was passed through the central lumen of the device, and the resected adenoma was caught (Fig. 4). The morcellation was performed by holding up the resected adenoma. After the morcellation, the small remnant resected adenomas were removed using the laparoscopic forceps. After removing all the adenomas, the bladder mucosa was closed under cystoscopic vision using a curved absorbable suture or a Carter-Thomason needle while maintaining the PNV (Fig. 5). At the end of the procedure, a 22F 3-way urethral Foley catheter was placed in situ for continuous postoperative irrigation. In general, the flow of the irrigation fluid was reduced gradually and was cutoff the next morning; the catheter was removed after confirming cessation of hematuria.
Port insertion under cystoscopy guidance. (A) An 11-mm laparoscopic self-retaining trocar (Transport, darim SurgNET Ltd., Seoul, Korea) was inserted 2 cm away from the pubic symphysis under cystoscopic guidance. (B) Establishment of the pneumovesicum while draining the normal saline.
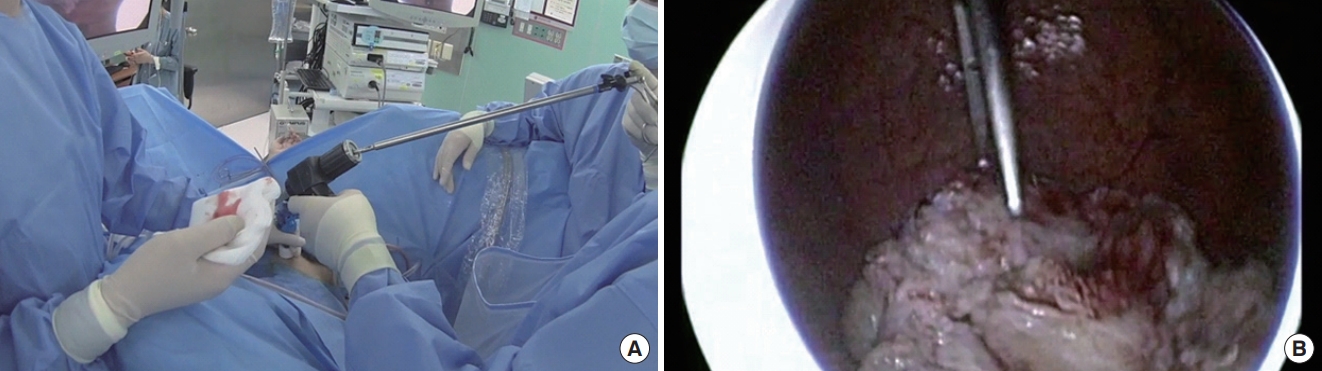
(A) Holding the resected adenoma with toothed forceps through the laparoscopic morcellator. (B) Holding the resected adenoma with toothed forceps under cystoscopic guidance.

(A) The morcellation was performed by holding up the resected adenoma. (B) Cystoscopic vision of morcellation with laparoscopic morcellator.
Definitions and Statistical Analysis
Morcellation time was defined as the total time needed for removal of the enucleated tissue. The time required for canister changes and vacuum reestablishment was not excluded from the morcellation time for the conventional urethral morcellator, nor was the time required to troubleshoot either device [11]. In the PNV group, morcellation time was calculated from the time of port insertion to wound closure. The enucleation and morcellation efficacies were calculated as the enucleated prostate tissue weight per enucleation time and morcellated tissue weight per morcellation time, respectively.
Subgroup analysis was performed to compare differences in perioperative outcome in patients with a large prostate (prostate volume >70 mL). Statistical analysis was performed using the Student t-test or the Wilcoxon rank-sum test for continuous variables, and the chi-square test for categorical variables. All data are reported as mean and range, considering P<0.05 as statistically significant. All the statistical analyses were conducted using IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA).
RESULTS
Twenty-one patients underwent successful laparoscopic PNV morcellation after HoLEP at the aforementioned institution. Table 1 summarizes the comparisons in the baseline characteristics and perioperative parameters between the transurethral morcellation (n=72) and PNV morcellation (n=21) groups. The transurethral morcellation group was significantly older (69.53 years vs. 67.29 years, P<0.05) and had a higher proportion with a history of hypertension than the PNV group. However, prostate volume was significantly larger in the PNV group (70.48 mL vs. 106.95 mL, P=0.030). The proportion of patients with a history of 5α reductase inhibitors administration, preoperative bacteriuria, concomitant bladder stones and an acute urinary state were higher in the PNV group. Regarding intraoperative parameters, enucleation efficacy (0.79 g/min vs. 0.89 g/min) and enucleation time (43.91 minutes vs. 59.19 minutes) were higher in the PNV group; however, there were no significant differences in these parameters between the 2 groups (P>0.05). In addition, there were no significant differences in the hospitalization period and occurrence of perioperative complications. However, tissue retrieval time (24.72 minutes vs. 7.81 minutes) and tissue retrieval efficacy (1.64 g/min vs. 8.50 g/min) were significantly different between the 2 groups (P<0.05).
Subgroup Analysis
A total of 67 patients with a prostate volume >70 mL were included in the subgroup analysis; 46 patients in the transurethral morcellation group and 21 in the PNV group (Table 2). There were no significant differences in the baseline characteristics, prostate volume, and preoperative IPSS score between the 2 groups, except for a history of hypertension and preoperative bacteriuria. However, enucleation time (68.46 minutes vs. 59.19 minutes, P=0.002), enucleation prostate weight (58.26 g vs. 65.48 g, P=0.001), enucleation efficacy (0.87 g/min vs. 0.89 g/min, P=0.012), tissue retrieval time (34.04 minutes vs. 7.81 minutes, P=0.001), and tissue retrieval efficacy (1.76 g/min vs. 8.50 g/min, P=0.001) showed significant between-group differences. Postoperative complications and hospitalization periods were similar (P>0.05).

Subgroup analysis (prostate volume >70 mL) of baseline characteristics of patients and perioperative parameters
At 1 month after surgery, both groups demonstrated a significant improvement in total IPSS, all subscores of the IPSS, including storage (IPSS questions 2, 4, 7), voiding symptoms (IPSS questions 1, 3, 5, 6), and quality of life (Table 3). In addition, all uroflow parameters, including maximal flow rate and the post voiding residual volume, improved significantly after surgery in both groups.
Of the 21 patients in the PNV group, 3 manifested a procedure-related complication. An extravesical leakage occurred in the 5th case during the PNV morcellation (Table 1). In this case, an abdominal distension was found due to irrigation fluid after the surgery. The extravesical leakage was seen in the cystography; the urethral catheter was maintained for 7 days. An additional cystography was performed before the removal of the urethral catheter to confirm that there was no extravesical leakage. Meanwhile, the 8th case developed a clot retention after removal of the urethral catheter, and the 9th case manifested failure of the self-voiding capacity after the removal of the urethral catheter on the 2nd postoperative day; hence, we inserted the catheter again and removed it on the 4th postoperative day.
DISCUSSION
The HoLEP has been proposed to treat large adenomas with similar efficacy and lower morbidity as compared to open prostatectomy [2,3]. However, the HoLEP has been reported in several studies as a technique for barriers to entry, it has not yet been implemented by the beginners. In addition, Peyronnet et al. [12] recently demonstrated that the learning curves for green laser enucleation of the prostate (GreenLEP) ranged from 14 to 30 cases, and for HoLEP it ranged from 22 to 40 cases. Since the invention of the mechanical tissue morcellator in 1996, various tissue morcellation systems have been tried. However, till date, the VersaCut morcellators have been used most widely [4]. VersaCut morcellators have an average retrieval efficacy of 1.0 to 5.6 g/min, as reported by most studies; however, there are differences among these studies [4,10,13].
In particular, the morcellation may delay the operation time due to the small visual field of the narrow scope, which may require an additional transurethral resection coagulation due to an unexpected injury. To overcome the limitations of the morcellation, several studies have been published comparing various morcellation methods. Chen et al. [14] have reported a method of reducing the operation time by effectively removing small adenomas that are difficult to catch during the morcellation using alligator forceps. Since fibrotic spherical glands with smooth surfaces and firm tissues can lead to difficulties in catching the pieces of adenomas and suctioning them, they reported a direct tissue removal method using a pair of forceps, which increased the morcellation efficacy up to an average of 7.3 g/min. In addition, in order to raise the efficacy of morcellation, the Richard Wolf Piranha was introduced with unique features designed to optimize this critical step of the procedure. The Richard Wolf Piranha performs rotating morcellation using serrated blades in which the prostatic tissue moves side to side. Tayeb reported that the Wolf Piranha showed a higher efficiency of 5.6 g/min by performing a randomized clinical trial comparing the Wolf Piranha and Lumenis VersaCut [9].
As mentioned above, small adenomas with a firm or smooth surface morphology may delay the surgery time [14]. In addition, the presence of dense prostatic tissue (colloquially referred to as “beach balls”) can also delay the morcellation time and increase the likelihood of bladder injury due to the morcellation [15]. Patients with frequent retention, frequent catheterization, or recurrent urinary tract infections may develop prostatic tissue inflammation leading to distorted architecture, resulting in an increased gland volume. Monn et al. [15] reported that inflamed prostate tissue may also cause increased bleeding or oozing whilst operating, which may result in poorer visualization requiring increased time to achieve an appropriate hemostasis during surgery.
Patients who underwent the procedure described in this report were those with poor visual acuity. In this case series, 47.6% of the patients had a history of catheterization, 33.8% of patients presented with a concomitant bladder stone, and 61.9% of the patients presented with bacteriuria in the preoperative urine culture. In our institution, the Lumenis VersaCut was used for the morcellation after the enucleation of the prostate. However, in some special situations, the visual field of the transurethral scope was poor or the morcellator was out of order and could not be used. Therefore, we were required to come up with an alternative morcellation method, and we found that this method has some advantages as follows:
First, the PNV allowed an excellent visual field. Even with oozing or bleeding, there was excellent visibility regardless of the prostate volume or amount of bleeding. Second, we could thoroughly remove all small adenoma tissues. Although there are not many reports, it is possible to miss a small floating adenoma tissue after the morcellation; this may require an additional cystoscopic intervention. Third, the current method has an excellent tissue retrieval efficacy, which has shown a greater efficiency than the traditional transurethral morcellation method with an efficiency of up to 5.0 g/min using a Lumenis Versa-Cut or up to 7.0 g/min using a Wolf Piranha. Fourth, since morcellation is performed by grabbing the resected adenoma with a grasping instrument passed through the central lumen of the morcellator, it prevents additional bladder injury that may occur during the removal of an adenoma. Since the morcellation is performed by pulling the tissue with a grasping instrument and not by inhalation of air or water, the possibility of bladder collapse or injury is low during the morcellation. Above all, the current method is easy to perform.
Two concepts were used to devise this method. First, procedures using a PNV can be applied to various urologic operations [16]. The PNV should provide excellent vision and should be free from bleeding or oozing in the bladder. Several previous researchers described the advantage of using laparoscopic instruments with the PNV in the removal of complex foreign bodies in the bladder [17-19]. Similar to the previous reports, we regarded resecting the adenoma as foreign body removal and applied this technique. Secondly, a laparoscopic morcellator presents a low risk of unexpected bladder injury during the morcellation. Most unpredictable bladder mucosal injuries occur when the bladder is collapsed. However, the laparoscopic morcellators do not cause bladder collapse without the suction of the CO2 gas. The laparoscopic morcellator is a device that is not familiar to urologists; however, the application of this equipment has recently been reported in some urologic diseases. Asimakopoulos et al. [20] reported on tissue retrieval performed using a laparoscopic morcellator in a case series of huge laparoscopic autosomal-dominant polycystic nephrectomies. However, this study was the first attempt to report tissue removal with a laparoscopic morcellator using a PNV.
This study has some limitations. First, enucleation time, enucleation prostate weight, and enucleation efficacy were shorter in the PNV group. The reason for this is because additional TUC before morcellation is often used in large prostate surgery due to the need for a good visual field to perform conventional morcellation. However, since this method is not affected by operative field oozing, the operation time can be shortened. In addition, when tissue retrieval was performed using this method, resected adenomas were not shredded and could be weighed without tissue loss.
In addition, this method has the limitation that it is not free from complications such as bowel injury or peritoneal leakage as in the case of suprapubic cystostomy insertion. In this series, the patient in the 4th case had urethral catherization for up to 7 days with a peritoneal leakage; however, there were no cases of bowel injury. In this case, a cystography was necessary to confirm that there was no extravasation. In addition, the current tissue retrieval method has a limitation of causing a wound of about 1 cm in the suprapubic area. Owing to these two critical issues, it is less likely that this method will be applicable to all patients. Nevertheless, this method can be considered as an alternative method that can be useful in situations when a transurethral morcellator is out of order or the morcellation time becomes inevitably extended due to bleeding or poor field of vision. In addition, the current method can be an alternative for an institution where Thulium-LEP or GreenLEP is performed, but it does not possess a transurethral morcellation equipment. If a thinner laparoscopic morcellator is developed in the future, a scar-less procedure using a 5-mm port will be possible.
In conclusion, a PNV morcellation using a laparoscopic morcellator is an effective alternative method for the retrieval of resected prostate tissues after HoLEP, as demonstrated in our case series. We introduced tips and tricks for this procedure to aid surgeons who cannot perform a transurethral morcellation after the HoLEP.
Notes
Research Ethics
This study was approved by the Institutional Review Board of Korea University Ansan Hospital (approval number: 2018AS0132). Because we retrospectively performed our investigation, the IRB waived the need for informed consent documents from our patients.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTION STATEMENT
· Full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis: BST, JHB
· Study concept and design: BST, JHB
· Acquisition of data: BST, HC
· Analysis and interpretation of data: BST, JHB
· Drafting of the manuscript: BST, BJJ
· Critical revision of the manuscript for important intellectual content: BJJ, HC, JYP, JHB
· Statistical analysis: BST
· Administrative, technical, or material support: JHB
· Study supervision: JHB