Emotional Stress Facilitates Micturition Reflex: Possible Inhibition by an α1-Adrenoceptor Blocker in the Conscious and Anesthetized State
Article information
Abstract
Purpose
To test the hypothesis that naftopidil prolongs intercontraction intervals in rats undergoing chronic stress as observed in previous animal models, voiding behavior and bladder function were measured and analyzed.
Methods
Female Sprague-Dawley rats weighing 200–230 g were exposed to repeated variate stress (RVS) for 1 week, chronic variable mild stress for 2 weeks, or simple mild stress for 1 week. Voiding behavior was assessed in metabolic cages. Voiding frequency and urine output were measured, and changes of these values were compared for the different types of stress. Micturition reflex was analyzed using unconscious cystometry. Naftopidil was administered orally at 30 mg/kg/day for 2 weeks.
Results
Unexpectedly, no stress-exposed rats exhibited increased micturition frequency compared to the normal nonstressed control. However, intercontraction intervals were shortened with each type of stress in the unconscious condition, especially by RVS (P<0.01). Naftopidil prolonged the shortened intervals.
Conclusions
Although voiding behavior appears approximately normal in rats chronically exposed to emotional stress, internal bladder function can be affected. With anesthesia, micturition intervals were moderately shortened by emotional stress and clearly improved by naftopidil. Therefore, naftopidil appears to act at the spinal level at least.
• HIGHLIGHTS
- Chronic emotional stress can alter urinary bladder function at the peripheral level by shortening intercontraction intervals.
- The central nervous system may suppress stress-associated bladder dysfunction.
- Naftopidil prolongs the intercontraction intervals shortened by stress.
INTRODUCTION
Micturition and emotion are closely linked [1]. Micturition is coordinated not in the spinal cord but in the brainstem, where it is closely associated with the limbic system [1]. As previous research indicated that psychotherapy improves nocturia and incontinence in patients with sensory urgency and detrusor instability [2], the association between mental state and lower urinary tract symptoms (LUTS) has been studied. Research indicates that the periaqueductal grey (PAG), pontine micturition center (PMC), and forebrain act in concert to modulate micturition during psychological stress [3-5]. The PMC, particularly Barrington’s nucleus, was identified as playing a pivotal role in coordinating forebrain and colonic/bladder pelvic viscera activity by bidirectional projections extending to the locus coeruleus and spinal cord [6,7].
The mechanisms of stress-associated voiding abnormalities have been investigated using experimental animal models. In male rhesus macaques subjected to social stress associated with the intruder paradigm, anxiety and fear-related behaviors such as urination were observed, and corticotropin-releasing hormone was found to play a crucial role in the physiologic response to psychological stress [8]. Neonatal maternal separation (NMS) induces an increase in voiding frequency and degranulation of mast cells in the urinary bladder, resulting in increased urinary bladder sensitivity compared to naive animals. However, compared to rats not exposed to exercise, voluntary wheel running attenuates voiding dysfunction and the activation of mast cell in rats subjected to NMS [9]. Water avoidance stress increases the micturition frequency in female rats but decreases it in male mice [10,11]. In female rats, the inflammation score and the number of degranulated mast cells in the bladder wall are higher compared with sham-treated rats [11]. Repeated variate stress (RVS) decreases the intercontraction interval, voided volume, and bladder capacity, although micturition pressure and baseline pressure are unchanged. RVS also increases the amounts of myeloperoxidase, nerve growth factor, and histamine in the urinary bladder [12]. Chronic variable mild stress (CVMS) increases the level of serum corticosterone and decreases the body weight in both males and females [13]. In the hypothalamic paraventricular nucleus, CVMS increases levels of corticotropin-releasing factor (CRF) mRNA in males and decreases CRF peptide in females; this factor coordinates brain noradrenergic activity and the sacral parasympathetic system [6].
The presence of LUTS in elderly men can be suggestive of benign prostatic hyperplasia, which presents with urgency, nocturia and hesitancy as the most bothersome symptoms [14]. To manage LUTS, α1-adrenoceptor antagonists are ordinarily prescribed, and these agents improve both voiding and storage symptoms. Animal experiments suggested that the mechanism of α1-adrenoceptor antagonists involves a decrement in urethral pressure and suppression of the micturition reflex [15,16]. Administration of the α1-adrenoceptor antagonists naftopidil intrathecally at the L6-S1 level suppresses the micturition reflex via modulation of glycine and GABA receptors [17]. Naftopidil improves shortening of intercontraction intervals in rat models of pelvic venous congestion, interstitial cystitis, bladder outlet obstruction, and cold stress [18-21]. Based on these data, we hypothesized that naftopidil also prolongs intercontraction intervals in rats subjected to chronic stress. To test this hypothesis, we exposed rats to three types of stress and assessed voiding behavior and urinary bladder function.
MATERIALS AND METHODS
Animals
A total of 77 female Sprague-Dawley rats weighing 200–230 g were used in the study. Rats were housed at a constant temperature (23℃±2℃) and relative humidity (55%±15%) under a regular light-dark schedule (light period 08:00 AM to 20:00 PM) with food and water freely available except during the time of the study tests.
Study Design
The design of the current study is summarized in Fig. 1. The study consisted of 3 sets of experiments involving different types of chronic stress. In each experiment, rats were divided into 4 groups (n=6–8 in each group): group 1, control without stress or naftopidil (α1-adrenoceptor blocker); group 2, administration of naftopidil without stress; group 3, subjected to stress but not treated with naftopidil; group 4, subjected to stress and treated with naftopidil. After the stress period, voiding behavior was assessed, and the micturition reflex was analyzed. The effect of naftopidil on various parameters related to urinary bladder function was also assessed.

Schematic illustration of the design of the current study. Rats were divided into 3 groups: chronic variable mild stress (CVMS), repeated variate stress (RVS), and simple mild stress (SMS). In each stress group, the rats were further divided into 4 subgroups: groups 1 through 4. After the stress period, voiding behavior was assessed and analyzed, followed by cystometry.
Chronic Stress Models
Table 1 summarizes the three different types of chronic stress applied in the study. First, rats were subjected to CVMS for 2 weeks, as described previously [13,22]. Rats were sequentially subjected to each of the following stressors: forced to swim at 4℃ for 2 minutes or at 12℃ for 4 minutes; exposed to humid sawdust for 3 hours, overnight, or 24 hours; fasted and deprived of water overnight or for 24 hours; conditioned to the light and dark cycle, and then isolated overnight or for 15 minutes at 4℃. Second, rats were exposed to RVS for 1 week, as described previously, with minor modifications [12]. Rats were sequentially subjected to each of the following stressors: oscillation at 60–120 rpm for 30 minutes in a plastic chamber secured on a rotator (SRR-2; AS ONE Corp., Osaka, Japan); forced swimming for 5 minutes in a cylinder filled with room-temperature water to a depth that prevented the tail from touching the bottom of the container; electrical foot-shocks (which make the rats jump promptly) twice for 5 seconds at 100 V with a 1-minute interval between shocks in a dedicated chamber box (custom built); restraint in a cylindrical device (9×15 cm [D×H]) (homemade) for 60 minutes. Third, rats were exposed to simple mild stress (SMS) consisting of lighting or humid sawdust for 24 hours every other day for 1 week. Rats were sequentially subjected to the following stressors: lighting overnight; humid sawdust for 24 hours every other day. After each stress period, voiding behavior and urinary bladder function were assessed.
Voiding Behavior
Rats were placed in metabolic cages (CL-0353; CLEA Japan Inc., Tokyo, Japan) with free access to standard food and water for 24 hours. The cumulative weight of urine produced by each rat was measured by the electronic balance (AD-1688; A&D Company, Ltd., Tokyo, Japan) and recorded on the connected computer (PC-LS150F2P2W; NEC Corp., Tokyo, Japan) each minute during the assessment. Voided urine volume and times were individually determined during the 12-hour dark period from 20:00 PM to 08:00 AM, the 12-hour light period from 08:00 AM to 20:00 PM, and the total 24-hour period.
Continuous Cystometry
Rats were anesthetized with urethane (0.4 g/kg intraperitoneally and 0.8 g/kg subcutaneously) and placed in restraining cages (NAIGAI-CFK-1P, NMS, Tokyo, Japan). A polyethylene catheter (PE-50; Clay Adams, Parsippany, NJ, USA) connected to an infusion pump was inserted transurethrally into the bladder of each animal. Physiologic saline was infused into the urinary bladder (0.05 mL/min), and bladder activity was monitored via the urethral catheter, which was connected to a pressure transducer and saline infusion pump via a 3-way stopcock. The bladder contraction interval, intravesical baseline pressure, and maximum bladder pressure were measured during the final 30–45 minutes of cystometry, which lasted at least 90 minutes.
Drug Administration
Naftopidil (Asahi Kasei Pharma Corp., Tokyo, Japan) was administered orally at 30 mg/kg/day via the food at 0.04% (w/w) for 2 weeks. In the case of CVMS, naftopidil administration was started at the beginning of the stress period. For week-long RVS or SMS experiments, naftopidil administration was started 1 week before the start date of the stress period.
Statistical Analysis
Results are shown as the mean±standard error of the mean. We primarily compared groups 1 and 3 and groups 3 and 4. In terms of multiplicity, data were analyzed using the Tukey-Kramer test. P<0.05 was considered indicative of statistical significance. All analyses were performed using JMP ver. 14 (SAS Institute, Cary, NC, USA).
RESULTS
Voiding Behavior
Data regarding voiding behavior after exposure to each type of stress are summarized in Table 2. In the CVMS model, the stress did not change the frequency of micturition between group 1 and 3 during the dark, light, or 24-hour periods. In the RVS model, the frequency of micturition of group 3 was significantly lower than that of group 1 during the dark and 24-hour periods (P<0.01 each). Also, the voided volume in group 3 was significantly lower than that of group 1 during the dark and 24-hour periods (P<0.01 each). In the SMS model, the stress did not affect the frequency of micturition in group 1 or 3 during the dark, light, or 24-hour periods.
Continuous Cystometry
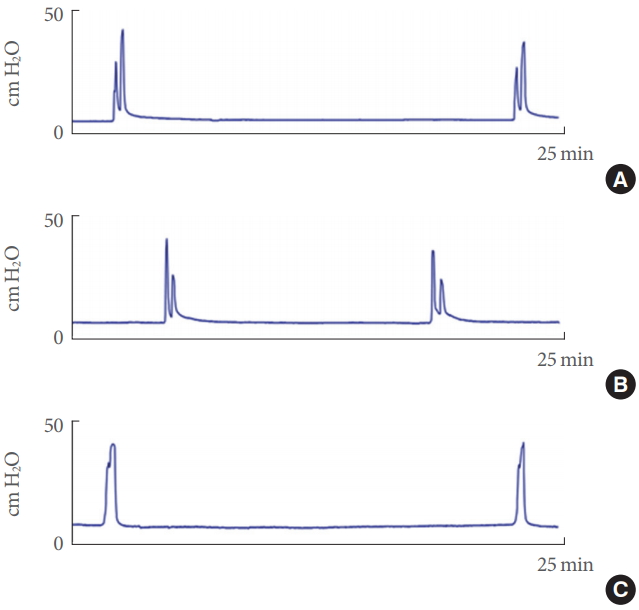
Data regarding unconscious cystometry after exposure to each type of stress are summarized in Table 3, and typical cystometrograms are shown in Fig. 2. In each of the stress models, the intercontraction interval tended to decrease in group 3 compared to group 1, and the difference between group 1 and 3 in the RVS model was statistically significant (P<0.01). Naftopidil significantly prolonged the decreased intervals in the RVS and SMS models (P<0.01 and 0.05, respectively) but not in the CVMS model. Baseline and maximum bladder pressure were not affected by any of the stress types examined.
DISCUSSION
In the present study, three types of stress were examined. Analysis of voiding behavior indicated that these stresses unexpectedly did not increase the micturition frequency, and RVS significantly decreased the micturition frequency during daytime and over the 24-hour period. However, cystometry experiments showed that the stresses examined, particularly RVS, significantly shortened the inter-contraction intervals. Naftopidil prolonged the decreased intervals.
Experimental Stress Model and Urinary Bladder Function
Previous research demonstrated that chronic stress modulates bladder function; water avoidance stress increases voiding frequency and shortens the voiding interval [23]. Social stress leads to urinary retention, decreased voiding frequency, and increases in voiding interval and urine volume [24,25]. RVS shortens the voiding interval and decreases the voided volume [12]. These data suggest that different types of stress lead to different dysfunctions in the urinary bladder. As a high micturition frequency generally indicates shortening of the intercontraction interval, there is a discrepancy in our present results between no change in voiding frequency (Table 2) and shortened contraction intervals (Table 3). The rather complex reasons for this discrepancy can be explained as follows. First, the central nervous system can strongly suppress stimulation of the micturition reflex as a mechanism for maintaining continence as the urinary bladder is filling, via neuronal projections connecting the PAG, PMC and forebrain [1]. Limbic structures prevent the PAG from exciting the PMC neurons, even when other parts of the PAG receive strong signals from the sacral cord that the urinary bladder is full and need to be emptied as soon as possible [26]. Medial frontal lobe neurons excited by glutamate inhibit the micturition reflex [27]. If this system operates under stressful condition such as those employed in the present study, and even if the emotional stress practically induces the micturition reflex, as indicated by Smith et al. [23], significant inhibition of the PAG and/or PMC may occur. Total voiding behavior is assumed to be balanced around normal micturition. This could explain why it appeared that micturition was not facilitated in group 3 as compared to group 1. We also speculate that together with enhancement of the micturition reflex brought on by emotional stress, activity of the central nervous system and/or limbic system can be increased to suppress the micturition reflex to maintain biophylaxis when in a conscious or awakened state. In our cystometry experiments, the animals were anesthetized; hence, the activity of the central nervous system and/or limbic system could have been suppressed, such that only the emotional stress had an effect on micturition frequency and voiding behavior. Regarding the condition of cystometry with the isovolumetric procedure under urethane anesthesia, MK-801 inhibits the reflex of bladder contractions, showing that glutamatergic excitatory mechanisms are essential for micturition [28]. MK-801 also facilitates micturition for initiation of the contraction reflex in unanesthetized decerebrate rats, indicating glutamate-mediated inhibitory control. These results at least partly support the above rationale. Secondly, the emotional stress could have been too weak and thus, inadequate for rats in experiments in a conscious state, as shortening of inter-contraction intervals was observed in the anesthetized animals. However, at least in the RVS model, as total urine output decreased in animals housed in metabolic cages (Table 2), we suspect that the emotional stress could have caused a depression-like state and consequent decrease in water intake. Therefore, speculation regarding weakness of the stressors can be excluded.
Experimental Stress Model and Changes in Bladder Tissue
In the present study of the effect of chronic stress on urinary bladder function, the micturition reflex was facilitated, even in anesthetized animals. This observation, supported by the results of previous studies, suggests that a functional change was generated in peripheral tissues, the urinary bladder, and/or the primary afferent nerve. In the urinary bladder, the number of mast cells and the inflammation score are increased by water avoidance stress [11]; histamine and myosin heavy chain are overexpressed; BrdU incorporation is increased by social stress [29]; and myeloperoxidase and nerve growth factor are upregulated by RVS [12]. In the hypothalamic paraventricular nucleus, CVMS increases expression of CRF mRNA in males and decreases expression of CRF peptide in females [13]. CRF is prominently expressed in the descending pathway from Barrington’s nucleus to the sacral parasympathetic nucleus (SPN) in the lumbosacral spinal cord, and prominent CRF immunoreactivity can be seen in the SPN of adult rats [30]. Therefore, CVMS may suppress the inhibition of micturition frequency by CRF at the lumbosacral spinal level.
Social stress was shown to increase both the intercontraction interval and bladder capacity. The mechanism involves upregulation of CRF mRNA for expression in Barrington’s nucleus, which projects to the pelvic organs such as the urinary bladder and colon, where CRF suppresses the activity of afferent nerves, resulting in enlargement of bladder capacity and the increment of intercontraction intervals [25,31]. Social stress elevates intracellular calcium levels, which in turn leads to an increase in the level of calmodulin-calcium complexes. Then, calcineurin dephosphorylates a nuclear transcription factor of activated T cells. As a result, changes in myosin isoforms in the urinary bladder and bladder fibrosis develop [31]. In contrast to social stress, repeated restraint-associated stress does not affect urinary bladder function [25]. Although the stresses examined in the present study shortened intercontraction intervals, the mechanism remains unclear. As unpredictable stress can alter gamma-aminobutyric acid-ergic (GABAergic) receptor expression [32], a change in GABAergic transmission may shorten the intervals in response to stress, particularly RVS.
Effect of Naftopidil on Bladder Function
During bladder filling, higher brain centers such as the prefrontal cortex suppress excitatory signals to the PMC to prevent abnormal voiding and incontinence [1]. Nishijima et al. [27] found that medial frontal lobe neurons excited by glutamate inhibit the micturition reflex via activation of the rostral pontine reticular formation via glutamatergic projections in conscious cystometry, whereas medial frontal lobe neurons excited by noradrenaline stimulate the micturition reflex. Micturition intervals are also prolonged by naftopidil administered in the medial frontal lobe instead of noradrenaline. Intrathecal administration of α1-adrenoceptor antagonists such as tamsulosin, silodosin, and BMY7378 prolongs micturition intervals [33]. These results suggest that naftopidil suppresses the micturition reflex at a higher brain center and/or spinal cord level.
The PMC projects directly into the parasympathetic bladder motor neurons and sacral GABAergic and glycinergic premotor interneurons that inhibit motor neurons in Onuf’s nucleus [1]. In anesthetized animals, intrathecal injection of naftopidil suppresses the micturition reflex in isovolumetric cystometry [16]. Abolishment of urinary bladder contractions by intrathecal administration of naftopidil is antagonized by simultaneous intrathecal administration of strychnine and/or bicuculine [17]. These results are supported by data from in vitro patch-clamp experiments in which naftopidil was shown to enhance inhibitory post-synaptic current in slices of lumbosacral spinal cord in rats [34]. These effects of naftopidil could be antagonized by strychnine or bicuculine as well. Therefore, bladder contraction caused by PMC stimulation may be suppressed by strengthening glycinergic and/or GABAergic input at the spinal level.
In conclusion, although voiding behavior may appear normal during chronic exposure to emotional stress, internal bladder function can be affected. Because the current results in terms of voiding behavior, which was assessed in the conscious state, were not ideal for evaluating the effect of emotional stress, we could not conclusively elucidate the mechanism of action of naftopidil in the brain center. In contrast, with anesthesia, micturition intervals were moderately shortened by emotional stress and definitely improved by naftopidil. This suggests that naftopidil acts at least at the spinal level. To treat LUTS associated with chronic emotional stress, enhancement of GABAergic or glycinergic input at the spinal level using naftopidil, for example, could be a viable treatment.
Limitations
Although several physiologic and behavioral parameters were assessed in the present study, experiments examining the effects of stress at the molecular level were not conducted. Future studies should thus evaluate the effects of chronic stress on urinary bladder function at the molecular level. Although urine volume was measured and discussed, the volume of water intake was not assessed in the present study.
Notes
Research Ethics
The study protocol (No. 5804) was approved by the President of the University of the Ryukyus based on the judgment of the institutional Animal Care and Use Committee.
Conflict of Interest
TH, the first author and corresponding author, belongs to Asahi Kasei Pharma Corporation. This study was supported by Asahi Kasei Pharma Corporation.
AUTHOR CONTRIBUTION STATEMENT
·Full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis: KS
·Study concept and design: KS
·Acquisition of data: SN
·Analysis and interpretation of data: SN, KS
·Drafting of the manuscript: TH
·Critical revision of the manuscript for important intellectual content: KS
·Statistical analysis: TH
·Obtained funding: KS
·Administrative, technical, or material support: TU, KK
·Study supervision: HY