Seasonal Variation of Overactive Bladder Symptoms in Female Patients
Article information
Abstract
Purpose
To evaluate seasonal variations of overactive bladder (OAB) symptoms in women who visited hospital clinics.
Methods
Medical records of female patients treated for OAB symptoms from January 2011 to December 2017 were retrospectively reviewed. Patients with pyuria at the first visit, those who did not complete the questionnaire, and those with <3 overactive bladder symptom scores (OABSS) were excluded. Uroflowmetric parameters, 3-day micturition diary, and OABSS were analyzed.
Results
A total of 582 patients with OAB symptoms who visited the hospital were enrolled in this study. Patients were grouped into 1 of the 3 season groups (cold, intermediate, and hot) depending on the average temperature of the month that the patient first visited the urologic department outpatient clinic. The total OABSS was significantly different between the 3 season groups (cold [7.25±3.20] vs. intermediate [6.24±3.40] vs. hot [5.51±3.20], P=0.001). The proportion of patients who had moderate OAB symptoms (6≤OABSS) was higher in the cold season group (56.2%) than in the other season groups (intermediate, 42.1%; hot, 31.8%; P=0.002). Differences in the number of micturitions (12.12±4.56 vs. 10.95±4.39, P=0.021) and number of urgent urinary incontinence episodes (2.06±0.94 vs. 2.48±0.87, P=0.001) between the cold and hot season groups were also significant. However, differences in the nocturia episode, total daytime voided volume, and mean voided volume between season groups were not significant.
Conclusions
Different urinary symptoms and uroflowmetric parameters were correlated with seasonal variation. OAB symptoms might be worse in cold season than in other seasons.
INTRODUCTION
The International Continence Society defined overactive bladder (OAB) as a symptom characterized by urinary urgency, with or without urinary incontinence, and by increased day-time frequency and nocturia if no proven infection or other obvious pathologies were observed [1]. The prevalence of OAB is high in both sexes [2]. According to a large international study, approximately 10.7% (4.3 billion) of the population worldwide in 2008 presented with OAB, and 546 million individuals are anticipated to present with OAB in 2018 [3].
The main pathological symptom of OAB is associated with abnormal bladder contraction during the filling phase of urination, leading to changes in other complex OAB symptoms [4]. This condition results from involuntary contractions that commonly caused by bladder outlet obstruction, stress urinary incontinence, aging, or possible idiopathies [5]. Because the majority of neurohumoral stimuli for bladder contraction is affected by acetylcholine-induced stimulation of postganglionic parasympathetic muscarinic cholinergic receptor sites in the bladder, most treatments for OAB target this action. Therefore, some researchers have revealed that agents (e.g., caffeine and nicotine) affecting acetylcholine stimulation may be associated with OAB symptoms [6,7].
Previous studies have presented the relationship between cold stress and detrusor overactivity in animal models. Among them, Imamura et al. [8-10] have performed a study on an animal model with detrusor overactivity induced by cold stress. Detrusor overactivity patterns were observed during the first 20 minutes of exposure to low room temperature in their animal study. However, during the second low-temperature exposure, detrusor overactivity patterns slowly mitigated and nearly disappeared. Finally, after 40 minutes of low-temperature exposure, micturition patterns of rats returned to baseline [11].
Some epidemiological studies have suggested that the prevalence of lower urinary tract symptoms (LUTS) is higher during winter than during summer based on cross-sectional studies, and animal studies have identified cold stress-induced detrusor overactivity [11-13]. However, the influence of environmental temperature on OAB in women has not been reported. Therefore, this study aimed to identify the impact of outdoor temperature on OAB symptoms in women without pyuria on urinalysis.
MATERIALS AND METHODS
The present study was approved by the Institutional Review Board of Korea University Ansan Hospital (IRB No. 2018AS0099) and conducted in accordance with the Declaration of Helsinki. Data collected from patients who visited the Department of Urology between January 2011 and December 2017 were retrospectively evaluated.
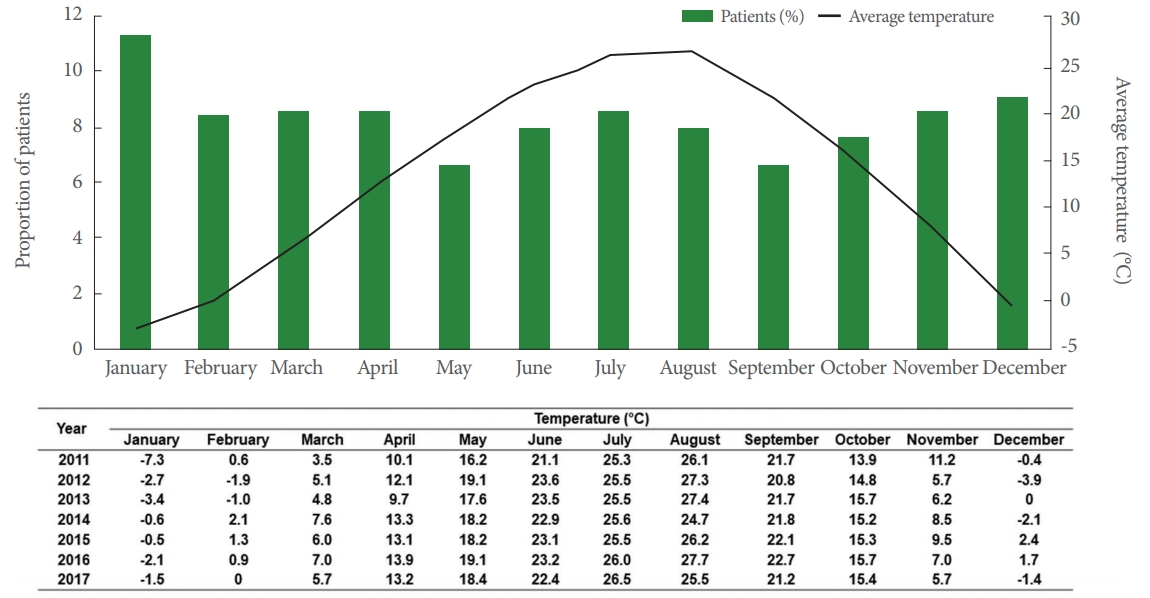
Ansan City, Korea, has a population of approximately 720,000 (women, approximately 348,000) and has 1-hour commuting distance from the city with the respective prefectural government. Ansan City has 4 distinct seasons, and winter season can be extremely cold with a minimum temperature of -20˚C. However, summer is hot and humid with temperatures exceeding 30˚C, and the average temperature in August ranged from 22˚C to 30˚C (http://countrystudies.us/south-korea/31.htm). The following data measured using the Korea Meteorological Administration (http://www.kma.go.kr) was used. The 4 coldest months were January, February, March, and December; the 4 hottest months were June, July, August, and September; and the intermediate months were April, May, October, and November. Patients were grouped into one of these 3 season groups depending on the average temperature of the month during the patient’s first visit to the urologic department outpatient clinic (Fig. 1).
Female patients aged ≥18 years with OAB symptoms (urinary frequency and urgency with or without incontinence) for ≥3 months were included in the analysis. Patients with urinary tract infection, recent history of pelvic surgery (within 2 months), urinary obstruction with indwelling catheter, neurologic disorders, or prolapse greater than POP-Q stage 2 and those who did not complete the questionnaire were excluded from the study. In addition, patients with <2 points for urgency score and <3 points for the overactive bladder symptom score (OABSS) were excluded from our analysis.
Current information was based on patient history and clinical examination during clinic visits. OABSS questionnaires (4 symptoms of OAB: daytime frequency, nighttime frequency, urgency, and urge incontinence) were used for the initial assessment of OAB symptoms and 3-day micturition diary (uroflow on subsequent visits). OABSS questionnaires and uroflow examination were evaluated in the first visit. Moreover, patients were instructed to complete the 3-day micturition diary for 3 days before the second visit.
Differences in symptoms and uroflow parameters and voiding pattern were separately examined according to season. Statistical comparisons were carried out using 1-way analysis of variance followed by 2-sided Tukey and Student t-tests. Data were analyzed using the IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA). A P-value of <0.05 was considered statistically significant.
RESULTS
The average monthly temperatures and proportion of patients who visited the Urology Department for OAB symptoms are described in Fig. 1. Patients most frequently visited the Urology Department with OAB symptoms in January and December, with smallest number of visits in May. However, no significant differences were observed in the number of patients visiting with OAB symptoms by season.
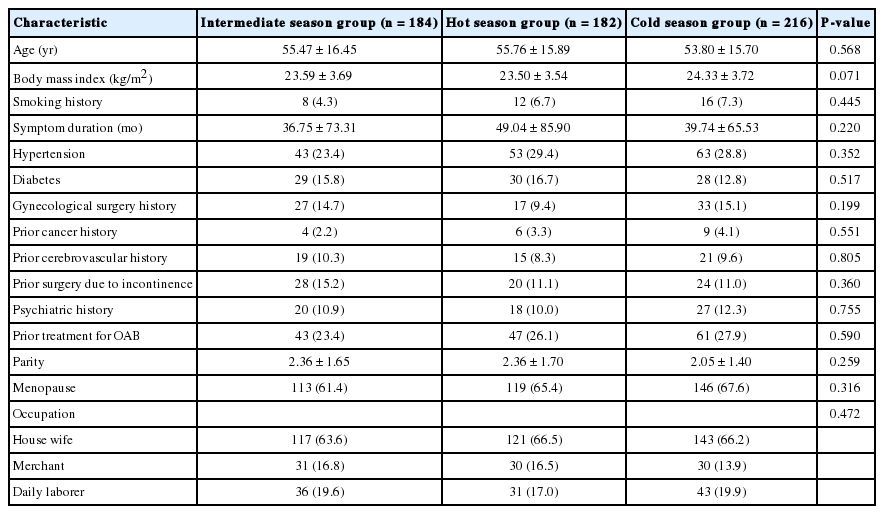
Patients’ baseline characteristics are shown in Table 1. The mean ages of participants in the intermediate, hot, and cold seasons were 55.47±16.45, 55.76±15.89, and 53.80±15.70, respectively. The mean body mass index (BMI) of the intermediate season group was 23.59±3.69 kg/m2, that of the hot season group was 23.50±3.54 kg/m2, and that of the cold season group was 24.33±3.72 kg/m2. These 3 groups did not significantly differ in terms of age and BMI. Moreover, the 3 groups did not significantly differ in terms of comorbidities (diabetes, hypertension, cancer, gynecological operation, menopause, and history of incontinence surgery), medication history (antipsychotic medication, prior treatment of OAB), and occupation.
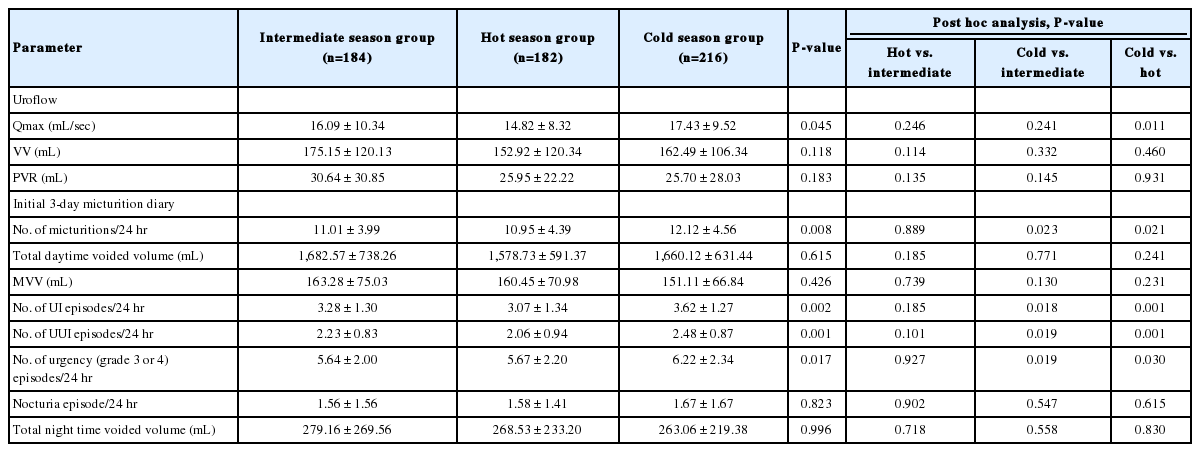
Table 2 shows the initial results of uroflow and 3-day micturition diary. Significant differences were found between 3 groups in daytime frequency, urinary incontinence (UI) episodes, urgency urinary incontinence (UUI) episodes, and number of urgency (grade 3 or 4) episodes. Patients in the cold season group especially showed more UI episodes (3.62±1.27 vs. 3.07±1.34, P=0.001), UUI episodes (2.48 ±0.87 vs. 2.06 ±0.94, P =0.001), day-time frequency (12.12 ±4.56 vs. 10.95 ±4.39, P =0.021) and number of urgency (grade 3 or 4) episodes (6.22±2.34 vs. 5.67±2.20, P=0.030) than those in the hot season group. In addition, the cold season group had a higher daytime frequency (12.12 ±4.56 vs. 11.01 ±3.99 , P=0.023), UI episodes (3.62±1.27 vs. 3.28±1.30, P=0.018), UUI episodes (2.48±0.87 vs. 2.23±0.83, P=0.019), and number of urgency (grade 3 or 4) episodes (6.22±2.34 vs. 5.64±2.00, P=0.019) than that in the intermediate season group. However, no significant differences were observed between the intermediate and hot season groups in the 3-day micturition diary. In addition, no significant differences were found between 3 season groups in the uroflow parameter, total daytime voided volume, mean voided volume, and number of nocturia.
A significant difference was observed in the total OABSS of the 3 groups (6.24±3.40 vs. 5.51±3.20 vs. 7.25±3.20, P=0.001) (Table 3). Three symptom scores of OABSS (daytime frequency, urgency, and urge incontinence) were higher in the cold season group than that in the hot and intermediate season groups. However, nighttime frequency symptom score of OABSS was not significantly different among the groups. The proportion of patients with moderate-to-severe symptoms based on the OABSS was higher in the cold group than in the hot and intermediate groups (cold [56.2%] vs. intermediate [42.1%] vs. hot [31.8%], P=0.002). However, no significant difference was observed in the proportion of patients with severe OAB symptoms (cold [12.3%] vs. intermediate [7.3%] vs. hot [8.2%], P=0.178).
DISCUSSION
Cold stress is associated with various physiological responses, and several studies are conducted to determine its association with various diseases. In particular, a previous study showed that cold stress affects the increase in plasma viscosity, serum cholesterol levels, blood pressure, sympathetic nervous activities, and platelet aggregation [14,15]. Moreover, in urologic diseases, acute urinary retention more commonly occurs in the morning, and daily temperature was associated with spontaneous acute urinary retention [16,17]. Similarly, OAB was more likely to occur during winter, and symptoms may also vary with temperature. However, studies regarding this issue are limited.
Numerous studies about the association between low temperature and urinary symptom focused on male patients. Because seasonal changes in urinary symptoms have been observed clinically, particularly in men, several researchers are focusing on symptoms, including benign prostate hyperplasia (BPH). Lee et al. [16] have revealed that the prevalence of spontaneous acute urinary retention was highest during winter based on a retrospective analysis. Choi et al. [18] also showed that cold temperature affected the urinary symptom score, particularly International Prostate Symptom Score (IPSS), voided volume (VV), and residual volume. However, Cartwright et al. [19] showed that the seasonal variation in frequency-volume parameters, symptom severity, or uroflowmetry parameters, except nocturia, was not observed in patients with benign prostatic hyperplasia, which is in contrast with results in a previous study. In addition, Watanabe et al. [20] suggested in their 5-year follow-up cohort study that total IPSS, quality of life (QoL), VV, or residual volume did not vary according to seasons.
However, in this study, the number of patients who visited during days with low temperatures and complained of urgency was higher. In addition, individuals who visited the hospital during winter had a higher number of voiding. This phenomenon can be explained based on the results in a previous study conducted by Mears and Shirreffs [21]. The result of study showed that sweat loss and water consumption were higher during warm days. The difference in these parameters may affect differences in OAB symptoms between groups.
In particular, studies that evaluated the effects of seasonal or temperature changes on OAB symptoms are limited. Nevertheless, these studies are controversial. Yoshimura et al. [12] in their community-based questionnaire study showed that winter season was an independent risk factor for frequency, urgency, and nocturia. However, Shim et al. [17] suggested in their crosssectional study that IPSS and OABSS were not affected by daily average temperature conditions. Previous epidemiologic studies mostly focused on normal populations, and these studies did not investigate parameters, such as voiding diary and uroflow. Therefore, consistent results are difficult to obtain. Furthermore, no study on the relationship between temperature and OAB symptoms in female patients has been conducted. To the best of our knowledge, this is the first study involving female patients with OAB.
In contrast to a previous study of male patients, the difference in uroflowmetry results between the season groups was not significant in this study. Previous researchers showed that cold stress increases sympathetic nervous system activity, which may cause the contraction of smooth muscles in the prostate, which in turn could lead to an aggravation of LUTS [22,23]. For this reason, studies on previous male patients may have affected the uroflowmetry results [18]. However, the difference between the subjective symptom and voiding diary outcomes was significant in women in this study as compared to the uroflow results.
In this study, the total OABSS of cold season group was higher than that of other season groups. This finding indicates that symptoms are more severe if patients visit the hospital on a cold day rather than on a warm day. In addition, patients had a higher frequency and urgency episode in their 3-day micturition diary on cold days. Some physiologic hypotheses state that low temperature is correlated with detrusor overactivity. First, the sympathetic nervous system activity associated with transient hypertension was partially correlated with cold stress-induced detrusor overactivity [11]. Second, the transient receptor potential (TRP) channel expression in the skin may be affected in individuals with active bladder symptoms. In a previous animal study, skin temperature was found to be associated with detrusor overactivity [11]. Cell bodies of temperature-sensitive neurons are located in the trigeminal ganglia and dorsal root ganglia (DRG). Moreover, TRPM8 channel proteins, which can be activated by low-temperature stimuli, are found in approximately 10%–15% of the trigeminal ganglia and 5%–10% of the DRG [24,25]. In a previous animal model study, TRPM8 channel knockout mice had significantly less cold sensitivity and almost no menthol sensitivity [26,27]. In addition, Shibata et al. [28] suggested that TRPM8 in the DRG might play a role in urinary urgency induced by cold sensation. Mukerji et al. [29] also suggested in their human study that cool and menthol receptor TRPM8 in the nerve fibers of overactive and painful bladders is associated with clinical symptoms.
Although there was no association with OAB symptoms in this analysis, few studies have shown differences in maximum flow rate (Qmax) on uroflow in terms of temperature. Cartwright et al. [19] depicted in a study of patients with BPH that uroflowmetric parameters were not associated with seasonal or mean monthly temperatures. Furthermore, Watanabe et al. [20] have presented Qmax differences in terms of seasonal changes, i.e., Qmax was higher in the cold season than that in the hot season (median, 9.4 mL/sec), which is similar to our results. However, this suggested the possibility that movement from a cold to warm place (examination room) contributed to increased Qmax at low temperatures. Movement causes the sympathetic nerve to be atonic, and as a result, individuals feel the urge to urinate.
The present study has some limitations. First, this retrospective study had a relatively small sample size. Second, cystometry and other urodynamic studies were not performed. Third, this study did not control all the possible influencing factors (for example; living environment) that could affect the outcome. In addition, setting the reference point of the season could have caused selection bias. However, since Korea has 4 seasons, the temperature was set as lower or higher than these seasons because the patient group ratio was not appropriate. Thus, a more well-designed prospective study on the measurement of temperature during the hospital visit or when writing a micturition diary in the home must be conducted. Despite the previously described limitations, our study has made several important contributions. The initial 3-day micturition diary and uroflow results of the present analysis can be utilized, unlike those of previous population-based studies. In addition, our study only included patients with a total OABSS score of ≥3. Therefore, cold stress was associated with symptom aggravation among patients with OAB.
In conclusion, different urinary symptoms and uroflowmetric parameters were correlated with seasonal variation. OAB symptom might be worse in cold season than in other seasons. Therefore, individuals must maintain a warm environment to prevent OAB. Future studies with larger sample size must be conducted to validate the results in the present study.
Notes
Grant/Fund Support
This research was supported by a grant from the Korean Continence Society and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2019R1F1A1058422).
Research Ethics
This study was approved by the Institutional Review board of the Korea University Ansan Hospital (IRB No. 2018AS0099).
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTION STATEMENT
·Full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis: BST, TYP, HC
·Study concept and design: BST, TYP, HC
·Acquisition of data: BST, TYP, HC
·Analysis and interpretation of data: BST, TYP, BJJ
·Drafting of the manuscript: BST, TYP
·Critical revision of the manuscript for important intellectual content: BJJ, JYP, JHB, HC, HC
·Statistical analysis: BST, TYP, YHL
·Obtained funding: HC, JHB
·Administrative, technical, or material support: HC
·Study supervision: HC