Efficacy and Safety of Naftopidil in Patients With Neurogenic Lower Urinary Tract Dysfunction: An 8-Week, Active-Controlled, Stratified-Randomized, Double-Blind, Double-Dummy, Parallel Group, Noninferiority, Multicenter Design
Article information
Abstract
Purpose
The aim of this study was to evaluate the efficacy and safety of naftopidil compared with tamsulosin in patients with neurogenic lower urinary tract dysfunction (LUTD).
Methods
This study was conducted as an 8-week, active-controlled, stratified-randomized, double-blind, double-dummy, parallel group, noninferiority, and multicenter clinical trial. After 2 weeks of screening, eligible subjects were randomly assigned to receive naftopidil (25 mg for 1 week followed by 75 mg for 7 weeks) or tamsulosin (0.2 mg for 8 weeks). Primary endpoint was a change of International Prostatic Symptom Score (IPSS) total score after 8 weeks of treatment.
Results
One hundred ninety-four subjects with neurogenic LUTD were included into this trial. There were no differences between the 2 groups in baseline characteristics, including urodynamic study results, subtype of LUTD, pretreatment and concomitant medication, and causes of neurogenic bladder. The medication compliance rate was 94.0% (naftopidil, 93.6%; tamsulosin, 94.4%). There was a statistically significant decrease of IPSS total score at 8 weeks versus baseline in both the naftopidil (-5.64±0.66) and tamsulosin (-6.53±0.65) groups (P<0.0001 each). The mean difference between both groups was 0.89 (upper limit of 95% confidential interval, 2.72), which was lower than the noninferiority limit of 3 points. A subgroup analysis of neurologic lesions and sex found no mean difference of IPSS total score in each group. There was also no difference in safety profiles, including treatment emergent adverse events.
Conclusions
Naftopidil was not inferior to tamsulosin as a therapeutic drug for patients with neurogenic LUTD and had a similar safety profile.
INTRODUCTION
Neurogenic lower urinary tract dysfunction (LUTD) is common in patients with various neurological diseases. Alphablockers have been shown to be effective treatments for improving storage symptoms as well as voiding symptoms in neurogenic LUTD [1,2]. Alpha1a-adrenergic receptors (ARs) predominate in the human prostate; blockade relaxes prostatic smooth muscle and increases urine flow [3], and α1d-ARs predominate in human detrusor, spinal cord, and afferent nerves, where blockade decreases LUTD symptoms. Thus, α1-AR act not only directly on the smooth muscle, but also on the spinal cord, ganglion and nerve endings affecting sympathetic and parasympathetic nerves [4]. Alpha1-blockers have been shown to reduce urinary tract resistance in patients with neurogenic LUTD and are effective in the treatment of voiding dysfunction, even in female patients [5].
Naftopidil was also reported to be effective on both symptoms and urodynamic variables of patients with neurogenic LUTD in a noncomparative, single-arm study [6]. Naftopidil is reported to have a 3-fold higher affinity for α1D-AR than α1AAR, while tamsulosin and silodosin have much more affinity for α1A-AR than α1D-AR [7,8]. Naftopidil appears to have similar effects on urological symptom scores and quality of life (QoL) compared to tamsulosin and silodosin in patients with nonneurogenic LUTD, such as benign prostatic hyperplasia [9]. Currently marketed α-blockers, including naftopidil, are being prescribed in patients with neurogenic LUTD in several countries. However, the clinical effects of naftopidil on neurogenic LUTD have been rarely reported. Therefore, we evaluated the efficacy and safety of naftopidil in patients with neurogenic LUTD.
MATERIALS AND METHODS
This study was conducted as an 8-week, active-controlled, stratified-randomized, double-blind, double-dummy, parallel group, noninferiority, and 15-multicenter clinical trial. The internal review board of each center approved this trial and written informed consent was obtained from all participants before screening. This study was registered in ClinicalTrials.gov (NCT02034604). The screening period was up to 2 weeks and the double-blind treatment period was 8 weeks.
This trial consisted of screening (visit 1), random assignment (visit 2), and follow-up at week 4 (visit 3), and week 8 (visit 4). After the screening test, eligible subjects were randomly assigned to 1 of 2 treatment groups (naftopidil or tamsulosin) by institution and sex at a ratio of 1:1. Subjects received either 25 mg of naftopidil for 1 week followed by 75 mg of naftopidil for 7 weeks, or 0.2 mg of tamsulosin for 8 weeks. International Prostatic Symptom Score (IPSS), uroflowmetry and postvoid residual (PVR) were conducted at screening visits 1, 3, and 4. Voiding diary was assessed at visits 2, 3, and 4, and the subject surveys (Benefit, Satisfaction, and Willingness to Continue Questionnaire [BSW-Q] [10], Global Response Assessment for Koreans [GRA-K], and Treatment Satisfaction Questionnaire [TSQ]) were administered at visits 3 and 4. The GRA measures the overall improvement with therapy. The assessment asks: “As compared to when you started the current study (treatment), how would you rate your overall lower urinary tract symptoms now?”. The patient was provided with the following 7 response options: markedly worse, moderately worse, slightly worse, no change, slightly improved, moderately improved, and markedly improved. The TSQ was rated on a 5-point scale, participant was asked: “overall how satisfied are you with your medication?”: very satisfied, somewhat satisfied, neither dissatisfied nor satisfied, somewhat dissatisfied, and very dissatisfied.
Inclusion and Exclusion Criteria
Qualified researchers confirmed and documented inclusion criteria for each patient. Inclusion and exclusion criteria are shown in Supplementary material 1.
Assessment
The aim of this trial was to evaluate the efficacy and safety of naftopidil compared to tamsulosin in patients with neurogenic LUTD. The primary endpoint of efficacy was to compare the change of IPSS total score (excluding QoL) after 8 weeks of treatment versus baseline. A secondary endpoint of efficacy was based on changes in baseline values for the following tests: uroflowmetry with PVR, IPSS subdomain, and QoL score, urinary frequency and nocturia, BSW-Q, GRA-K, and TSQ. Safety endpoints consisted of treatment emergent adverse events (TEAEs), adverse drug reactions (ADRs), and serious AE/ADR. All adverse events were classified according to WHO Adverse Reaction Terminology (WHO-ART092).
Statistical Methods
The change in the 8-week IPSS total score versus baseline was projected to be approximately 8 to 9 in the 2 treatment groups and the standard deviation was projected to be approximately 7. The noninferiority limit of naftopidil for tamsulosin was defined as 3, which was assumed to maintain about 60%–70% of the tamsulosin effect regarding the change in 8-week IPSS total score versus baseline. Under this assumption, the sample size for the evaluation of noninferiority at 80% power and 2.5% onesided significance level was 172 cases, i.e., 86 cases per treatment group. A total of 204 subjects were recruited with 102 cases per treatment group considering a 15% dropout rate. For primary endpoint, to demonstrate noninferiority of naftopidil to tamsulosin, if the upper limit of 2-sided 95% confidence interval (CI) for differences in least square means between 2 treatment groups (naftopidil-tamsulosin) calculated using an analysis of covariance (ANCOVA) model in which baseline values were used as a covariate was less than noninferiority margin of 3 points, then naftopidil was considered to be noninferior to tamsulosin. For efficacy assessment, an ANCOVA model with baseline values as a covariate was used to compare the endpoints between treatment groups for continuous data and the chi-square test or Fisher exact test was used for categorical data. For safety assessment, frequencies and percentages of subjects who experienced AEs were summarized and the difference of incidence rate between treatment groups was compared using the chisquare test or Fisher exact test. Statistical analysis was performed using the SAS ver. 9.2 (SAS Institute, Cary, NC, USA).
RESULTS
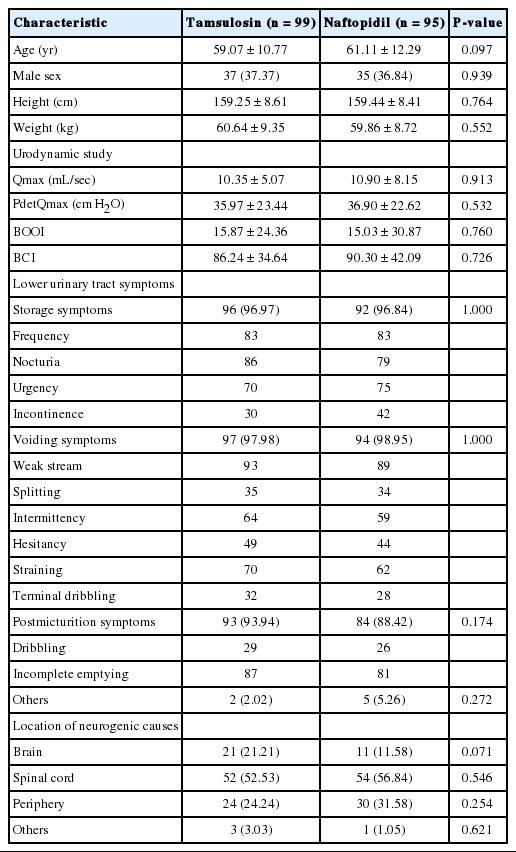
Baseline characteristics of the 194 subjects enrolled in this trial are presented in Table 1. There were no differences between the 2 groups in pretreatment characteristics such as urodynamic study results, type of LUTS, and causes of neurogenic bladder. The medication compliance rate was 94.0% for the entire study group, 93.6% for the naftopidil group, and 94.4% for the tamsulosin group, for a total of 194 patients included in the intent-to-treat set consisted of all randomized subjects.
Of the 194 randomized subjects (95 naftopidil and 99 tamsulosin), 181 (89 naftopidil and 92 tamsulosin) who received at least one dose of clinical trial medication and could be evaluated for the primary efficacy endpoint after randomization were included in the full-analysis (FA) set. The safety analysis group was defined as at least one dose of the clinical trial drug and one or more safety-related evaluations by telephone or visit after drug administration. Of the 194 randomly assigned subjects, 189 who received a clinical trial drug (92 naftopidil and 97 tamsulosin) were included in the safety analysis group, excluding 5 (3 naftopidil, 2 tamsulosin) (Fig. 1).
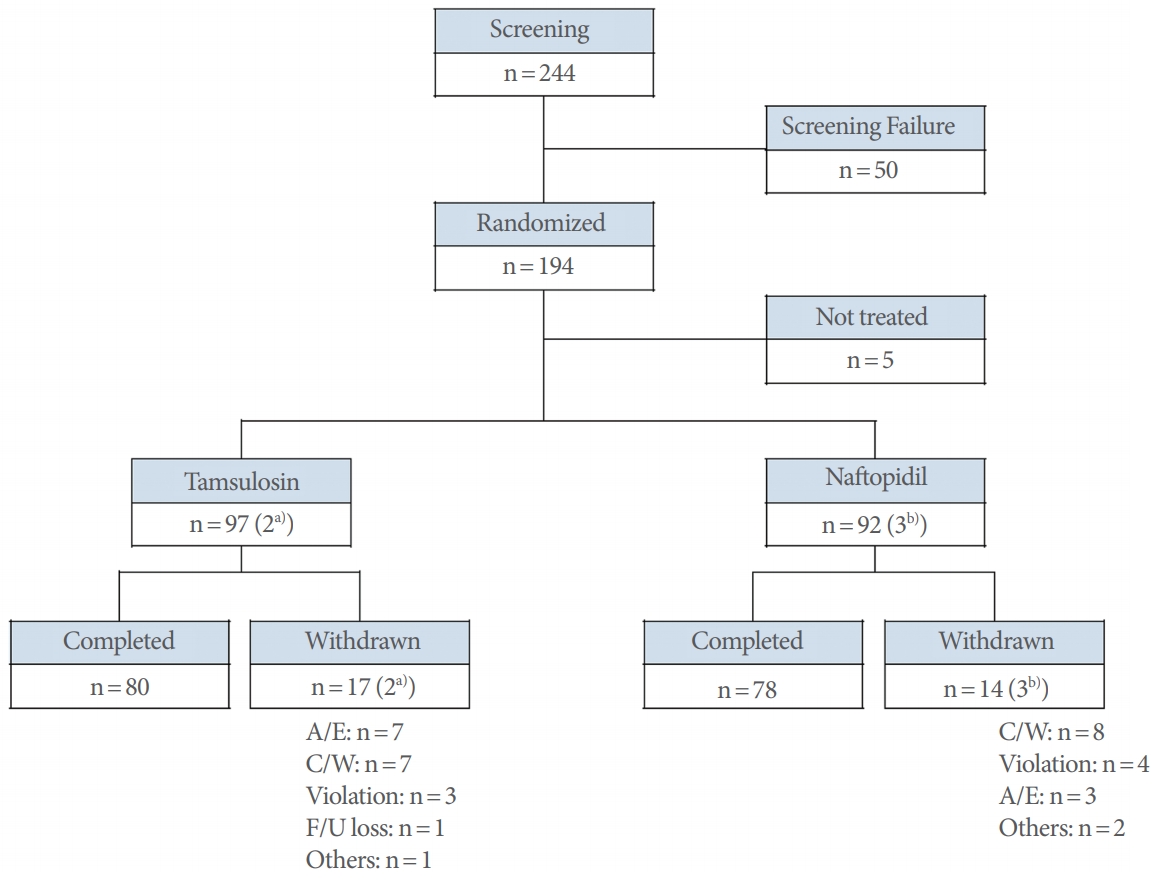
Flow chart of the study. A/E, adverse events; C/W, consent withdrawal; F/U, follow-up. a)C/W: n=1; Violation: n=1. b)C/W: n=2; Others: n=1.
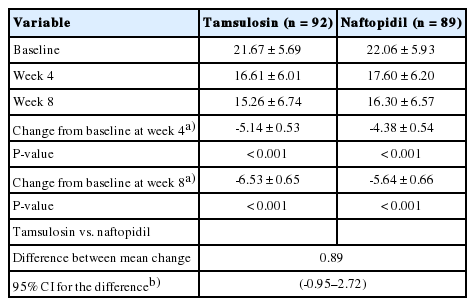
Both groups showed a statistically significant decrease in the FA set of IPSS total score at 8 weeks (mean change of the naftopidil group, -5.64±0.66; tamsulosin group, -6.53±0.65, respectively, P<0.0001). The mean difference between both groups was 0.89, and upper limit of 95% CI for the difference between the 2 groups was 2.72, which was lower than the noninferiority limit of 3 points. Therefore, compared with tamsulosin, naftopidil was found to be noninferior in IPSS total score change at 8 weeks (Table 2).
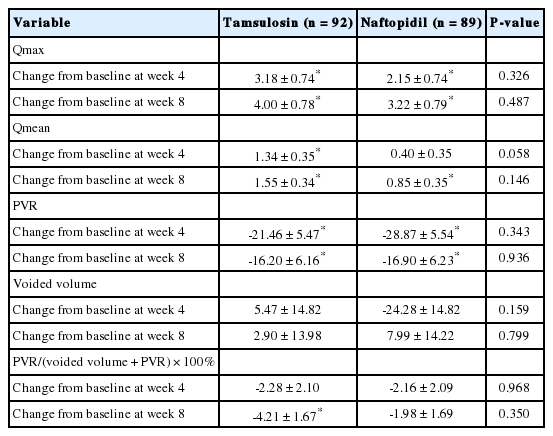
For secondary endpoints, all variables of uroflowmetry and PVR parameters (Table 3), IPSS subdomain and QoL score (Fig. 2), urinary frequency and nocturia (Fig. 3) at 4 and 8 weeks were not significantly different between the tamsulosin and naftopidil groups. There were also no differences between groups at 8 weeks in the BSW-Q, GRA-K, and TSQ. There were significant differences between the tamsulosin and naftopidil groups in the response distribution of the GRA-K (P=0.0318) and TSQ (P= 0.0095) at 4 weeks.
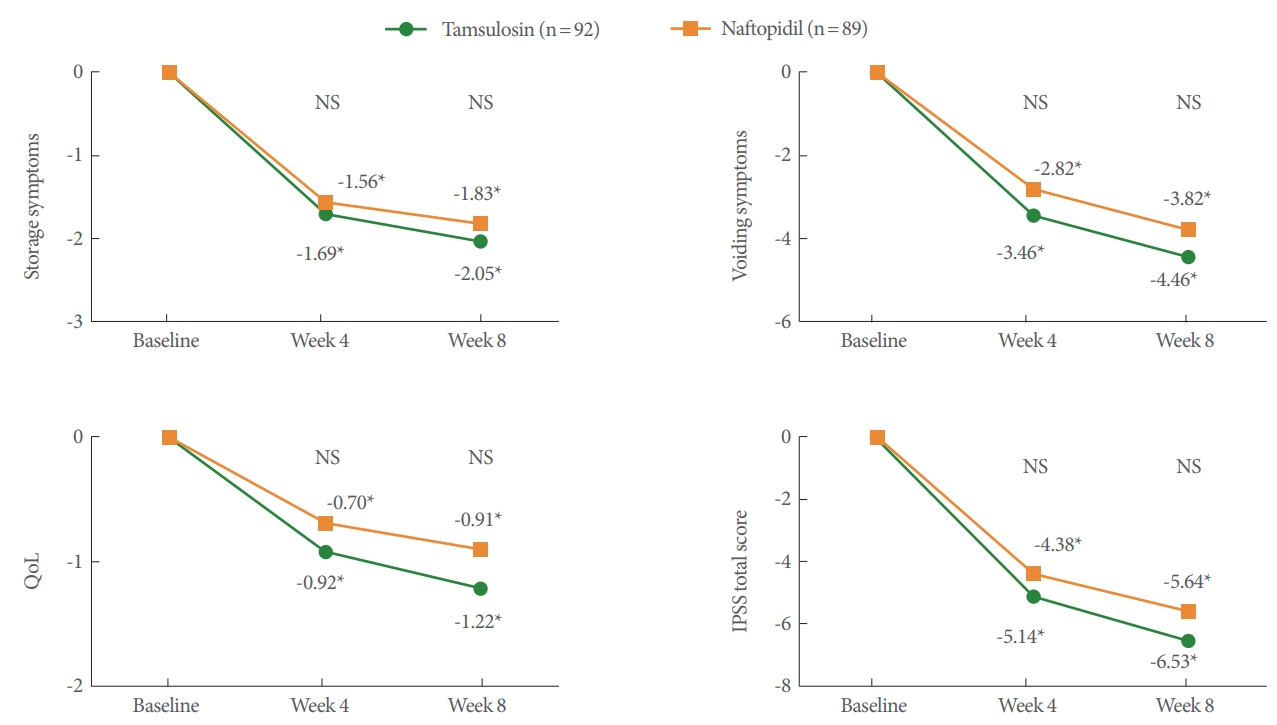
Change from baseline to 4 and 8 weeks in International Prostatic Symptom Score (IPSS) and quality of life (QoL) score. *Statistically significant change from baseline. NS, statistically nonsignificant change between the tamsulosin and naftopidil group.
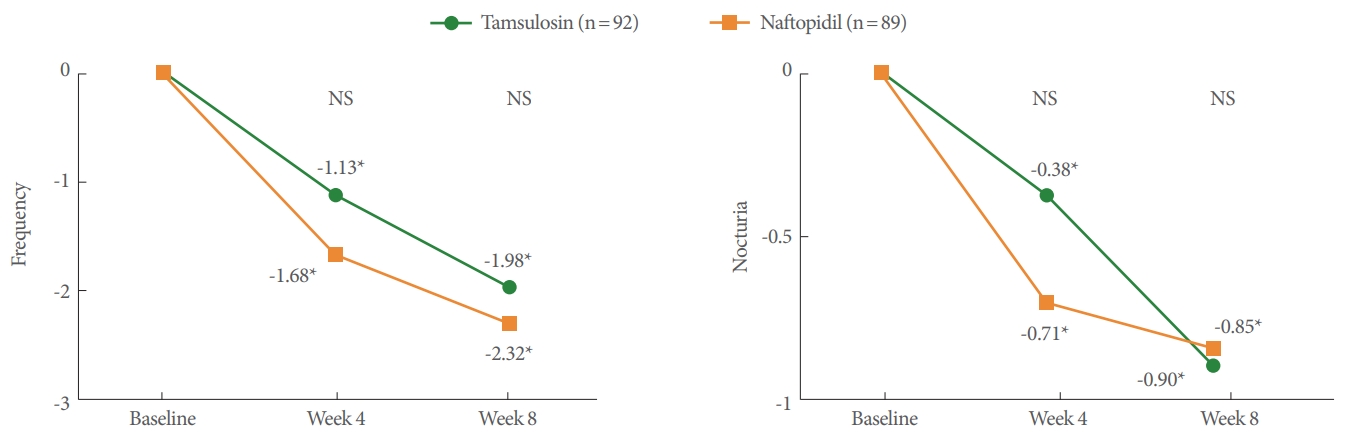
Change from baseline to 4 and 8 weeks in urinary frequency and nocturia. *Statistically significant change from baseline. NS, statistically nonsignificant change between the tamsulosin and naftopidil group.
The subgroup analysis of neurologic lesions showed that there was no mean difference (naftopidil group - tamsulosin group) in IPSS total score change at 8 weeks between the 2 treatment groups. The mean difference of IPSS total score was -0.79 (95% CI, -6.02 to 4.43) in subjects with brain lesions, 0.63 (-1.86 to 3.13) in subjects with spinal cord problems, and 2.51 (-1.16 to 6.17) in subjects with peripheral nervous disease. There was no difference between the 2 groups according to sex. The mean difference of the IPSS total score change at 8 weeks between the 2 treatment groups was -0.36 (-3.52 to 2.81) for male subjects and 1.51 (-0.81 to 3.84) for female subjects.
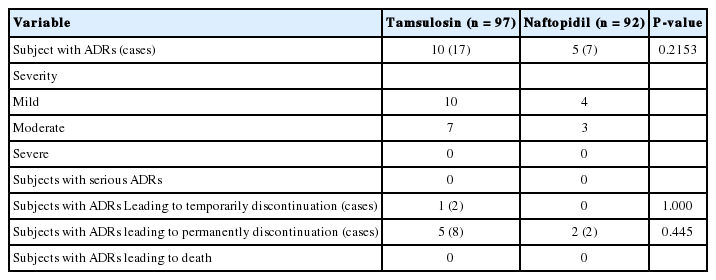
During the trial, 97 TEAEs occurred in 57 subjects (30.16%) of the 189 subjects for safety analysis set, including 27 subjects (29.35%, 42 cases) in the naftopidil group and 30 subjects (30.93%, 55 cases) in the tamsulosin group, and there was no difference of incidence rate between the treatment groups (P= 0.8130). Eleven serious AEs occurred in 9 subjects (4.76%), 2 with naftopidil (2.17%, 4 cases), and 7 with tamsulosin (7.22%, 7 cases). There was no difference of incidence rate of serious AEs between the treatment groups (P=0.1705). Five AEs that resulted in temporary discontinuation of the medication occurred in 2 subjects (1.06%), 1 in the naftopidil group (1.09%, 2 cases), and 1 in the tamsulosin group (1.03%, 3 cases). There was no difference of incidence rate of AEs which resulted in temporary discontinuation of the medication between the treatment groups (P=1.0000). Three patients (3.26%, 6 cases) in the naftopidil group and 7 patients (7.22%, 10 cases) in the tamsulosin group needed to discontinue medication permanently (P=0.3322). No AE-related death was reported during this trial. ADRs are shown in Table 4. Ten ADR cases that resulted in permanent discontinuation of the drug were reported in 7 patients, including dyspepsia (n=1) and nausea (n=1) in the naftopidil group, and urinary incontinence (n=2), urinary frequency (n =1), abdominal pain (n =1), dizziness (n =1), tremor (n=1), pruritus (n=1), and skin rash (n=1) in the tamsulosin group. Of these, all of the ADRs that resulted in permanent discontinuation of naftopidil were mild or moderate, and all resolved without sequelae except urinary incontinence (n=1), urinary frequency (n=1) in the tamsulosin group. There were no AEs that resulted in serious ADRs or death in this trial, and no other specific finding was found in other safety profiles. Therefore, it was confirmed that the safety profile of naftopidil did not differ from that of tamsulosin.
DISCUSSION
The aim of this trial was to evaluate the efficacy and safety of naftopidil in patients with neurogenic LUTS. After 8 weeks of administration, both the naftopidil and tamsulosin groups showed statistically significant improvement in the primary efficacy variable of total IPSS, and naftopidil was not inferior to tamsulosin in terms of improved total IPSS. In addition, there was no significant difference between the tamsulosin and naftopidil groups in all variables at 4 and 8 weeks in uroflowmetry and PVR changes or questionnaires about urinary symptom changes. There were significant differences between the tamsulosin and naftopidil groups in the GRA-K (P=0.0318) and TSQ (P=0.0095) at 4 weeks. However, when the results were subdivided into 3 categories (improvement, no change, or deterioration for the GRA-K, and satisfaction, no satisfaction and no dissatisfaction, or dissatisfaction for the TSQ), there was no difference between the groups even at 4 weeks.
Alpha-blockers may play an important role in improving emptying by decreasing outlet resistance in neurogenic LUTD. Alpha-blockers decrease PVR and increase urinary flow rate in patients with a neurogenic bladder [11,12]. Kakizaki et al. [11] reported that tamsulosin reduces functional urethral resistance during voiding and improves the flow rate in patients with a neurogenic bladder. Alpha-blockers have also been shown to decrease detrusor overactivity on urodynamic studies [13] and have beneficial effects on storage symptoms [14]. In patients with a neurogenic bladder and poor bladder compliance, combination medical therapy including α-blockers significantly improves compliance, decreases bladder pressure at capacity and improves clinical outcomes compared to single antimuscarinic therapy [15,16]. Collectively, α-blockers have some modest effects in patients with a neurogenic bladder in facilitation of voiding, but also serve to increase capacity and compliance in combination with anticholinergics [17]. In addition, α-blockers have some effects on preventing serious harm from autonomic dysreflexia [2,12,18]. Krum et al. [19] reported that α-blockers significantly decrease the magnitude of hypertension and severity of secondary symptoms during autonomic dysreflexia triggered by a variety of iatrogenic stimuli.
Although α-blockers have shown some clinical effects on neurogenic bladders, the precise mechanisms of action are still unknown. Abrams et al. [1] found that tamsulosin decreases maximum urethral pressure in patients with neurogenic LUTD. Bladder outlet obstruction can be reduced by a decrease in the maximum urethral pressure in patients with spinal cord injury (SCI) [20]. Regarding the expression of α1-ARs in the human spinal cord, α1AR mRNA is predominantly present in ventral gray matter (ventral>dorsal; sacral>lumbar=thoracic>cervic al), while α1D-AR is the predominant receptor subtype overall [21]. Traditionally, α1D-ARs are known to be mainly expressed in the bladder detrusor, with higher mRNA expression than that of α1A-Ars [22,23]. Thus, α1D-ARs are believed to be closely involved in storage symptoms. In a recent report using a decerebrated rat model with SCI, Ishida et al. [24] measured sphincter electromyography activity and demonstrated that naftopidil increased voiding efficiency by reducing external urethral sphincter resistance, while silodosin did not affect any parameters. The authors concluded that α1D-AR plays an important role in maintaining external urethral sphincter activity in the spinal cord, and α1D-blockade may influence the activity of Onuf’s nuclei motor neurons, resulting in a decrease in external urethral sphincter tone.
Despite these data, the clinical effects of naftopidil on neurogenic LUTD have been rarely reported. A Japanese naftopidil neurogenic LUTD study group demonstrated that naftopidil has a significant effect on both symptoms and urodynamic variables of patients of both sexes with neurogenic LUTD [6]. They also found that PVR<300 mL and bladder contractility are predictive factors for the efficacy of naftopidil. Theirs was the first report regarding the effect of naftopidil in neurogenic LUTD, although it was not a comparative study. In the current study, naftopidil improved subjective symptoms and objective findings in patients with neurogenic LUTD, and its effects were not inferior to those of tamsulosin. These results were effective regardless of sex or neurological causes. To our best knowledge, this is the first randomized controlled study to compare naftopidil and the other subtype of α1-blockers (e.g., tamsulosin) in neurogenic LUTD.
This study has several limitations. First, when managing neurogenic LUTD in real clinical practice, it is much more common to use a combination of medications including anticholinergics and β3-agonist than α-blockers alone. The trial treatment strategy may not fit well with actual clinical situations. Second, there was no evaluation of long-term outcomes, given the follow-up period of only 8 weeks. Although the follow-up period was short, it was considered sufficient to compare the effects and side effects of the 2 drugs.
In conclusion, this clinical trial demonstrated that naftopidil was not inferior to tamsulosin as a therapeutic drug for patients with neurogenic LUTD. Naftopidil had a safety profile similar to tamsulosin and should be considered as a safe drug that can improve symptoms in patients with neurogenic LUTD.
SUPPLEMENTARY MATERIALS
Supplementary material 1 can be found via https://doi.org/10.5213/inj.1938198.099.
Acknowledgements
The following investigators also participated in the study: Jong Bo Choi, Ajou University School of Medicine, Suwon; Hyeong-Gon Kim, Konkuk University Medical Center, Seoul; Jeong Gu Lee, Korea University College of Medicine, Seoul; SeungJune Oh, Seoul National University Hospital, Seoul; Seong Jin Jeong, Seoul National University Bundang Hospital, Seongnam; Ju Tae Seo, Cheil General Hospital & Women’s Healthcare Center, Seoul; Ha Na Yoon, Ewha Womans University, Seoul; SunOuck Kim, Chonnam National University Hospital, Gwangju; Khae Hawn Kim, Gachon University Gil Medical Center, Incheon; Jeong Zoo Lee, Pusan National University Hospital, Busan; Tae Yoong Jeong, Myongji Hospital, Goyang, Korea.
Notes
Research Ethics
The Internal Review Board of each center approved this trial and written informed consent was obtained from all participants before screening. This study was registered in ClinicalTrials.gov (NCT02034604).
Conflict of Interest
This study was sponsored by Dong-A ST Co., Ltd., Seoul, Korea and Asahi Kasei Pharma Co., Ltd., Tokyo, Japan. No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTION STATEMENT
·Conceptualization: KSL
·Formal Analysis: HHS, KSL
·Investigation: HHS, MSC, JCK, JHK, KSL
·Methodology: MSC, JCK, JHK, KSL
·Project Administration: MSC, JCK, JHK, KSL
·Writing – Original Draft: HHS
·Writing – Review & Editing: MSC, JCK, JHK, KSL