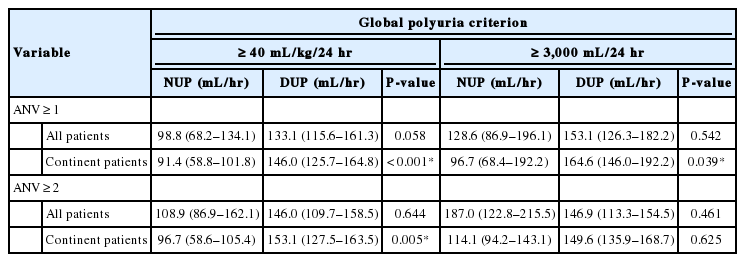
Nocturnal Urine Production in Women With Global Polyuria
Article information
Abstract
Purpose
Low nocturnal urine production (NUP) may be sufficient to rule out global polyuria (GP) in men. This study determines the sensitivity of indices for nocturnal polyuria (NP), defined as nocturnal polyuria index (NPi; nocturnal urine volume/24-hour urine volume) ≥0.33 or NUP ≥90 mL/hr, for detecting GP in women.
Methods
Data were analyzed from 2 prospective protocols involving subjects recruited from a urology ambulatory care unit and a continence clinic. Women ≥18 years with nocturia were included if they met either of 2 common criteria for GP: (1) ≥40 mL/kg/24 hr or (2) ≥3,000 mL/24 hr.
Results
Thirty-one women were included (NPi, 28.6 [21.3–40.7]; NUP, 100.8 [68.3–135.8] mL/hr). At the ≥40 mL/kg/24-hr cutoff, 40% and 63% of women reporting ≥1 nocturnal void(s) (n=30) had NPi ≥0.33 and NUP ≥90 mL/hr, respectively. Additionally, 53% and 71% of subjects reporting ≥2 nocturnal voids (n=17) had NPi ≥0.33 and NUP ≥90 mL/hr, respectively. At the ≥3,000 mL/24-hr cutoff, 38% and 69% of women reporting ≥1 nocturnal void(s) (n=13) had NPi ≥0.33 and NUP ≥90 mL/hr, respectively, and 63% and 88% of subjects reporting ≥2 nocturnal voids (n=8) had NPi ≥0.33 and NUP ≥90 mL/hr, respectively. By extension, 37%–62% of women with nocturia and GP did not have NP by NPi ≥0.33 criteria, and 12%–37% did not have NP by NUP ≥90 mL/hr criteria.
Conclusions
Indices of excess nighttime urination do not reliably predict GP in women. A full-length voiding diary may be particularly important in the evaluation of women with nocturia. Nocturia in women merits further consideration as a distinct entity.
INTRODUCTION
Nocturia, defined by the International Continence Society as waking to pass urine during the main sleep period [1], involves a fundamental mismatch between production and storage. This mismatch may be mediated by excess urine production (i.e., nocturnal polyuria [NP] or global polyuria [GP]) and small bladder capacity [2]. Voiding diaries are the gold standard for differentiating these mechanisms [3], but many patients find them prohibitively cumbersome [4], such that voiding diary utilization remains suboptimal [5].
Nocturnal-only voiding diaries have been proposed as an alternative to voiding diaries of longer duration [6], and likewise offer valuable insight regarding nocturnal urine volumes and bladder capacities, which can be used to support diagnoses of NP and nocturnal small bladder capacity, respectively. In contrast to NP and small nocturnal bladder capacity, a diagnosis of GP is, by definition, predicated on 24-hour urine sampling [1,7]. Nevertheless, it stands to reason that inordinate nocturnal urine volumes are highly suggestive of GP, and provide a strong indication for a full-length voiding diary. Recent research demonstrated that a high nocturnal urine volume among men with NP is fairly sensitive for a diagnosis of comorbid GP [8]. However, the relationship between nocturnal urine production (NUP) and 24-hour urine production remains poorly characterized in women with GP. Accordingly, the aim of the present study is to determine the sensitivity of standard indices for NP in detecting GP in women.
MATERIALS AND METHODS
1. Study Protocol
The present study is a post hoc analysis of voiding diaries obtained from 2 prospective observational protocols. The first protocol enrolled subjects (n=135) recruited by a urology ambulatory care unit [9]. The second protocol (n=95) recruited participants ≥65 years who consulted a continence clinic [10]. For both protocols, participants completed 24- to 72-hour voiding diaries, and subsequently collected urine samples at 3-hour intervals to complete renal function profile. Both studies were conducted following local ethics committee approval in accordance with the Declaration of Helsinki on an a priori basis. The methodology of these studies has previously been described [9,10].
2. Subjects
Voiding diaries were analyzed to identify subjects with GP using 2 distinct criteria for GP: (1) 24-hour urine production ≥40 mL/kg and (2) 24-hour urine production ≥3,000 mL [1,7]. Women ≥18 years of age with nocturia were included if they met either criteria for GP.
3. Voiding Diary Parameters
Twenty-four-hour urine volume was defined as the total quantity of urine passed in one day. The actual number of nocturnal voids (ANV) was defined as the number of nocturnal awakenings during the intended sleep period. Nocturnal urine volume was defined as the quantity of urine produced during the period of intended sleep (including the first morning voided volume) [1]. Nocturnal polyuria index (NPi) was defined as the fraction of 24-hour urine output produced during the intended sleep period (nocturnal urine volume/24-hour urine volume) [1]. NUP was defined as the hourly rate of urine production during the intended sleep period (nocturnal urine volume/sleeping hours) [1]. Daytime urine production (DUP) was defined as the rate of urine produced during nonsleeping hours [(24-hour urine volume – nocturnal urine volume)/(24 – sleeping hours)].
4. Test Methods
For each GP criterion (i.e., ≥40 mL/kg/24 hr and ≥3,000 mL/24 hr), the sensitivity of indices for NP in diagnosing GP was determined using 2 common criteria for NP: (1) NPi ≥0.33 and (2) NUP ≥90 mL/hr [1,11]. DUP was also calculated for each GP-NP criteria combination to further characterize urine production by circadian period (i.e., nighttime vs. daytime).
A subgroup analysis excluding patients with nocturnal urinary incontinence was performed to account for potential differences by continence status. Nocturnal incontinence was identified by one or more nighttime incontinence episodes on voiding diary analysis.
5. Statistical Analysis
A Wilcoxon signed-rank test was used to compare NUP versus DUP for matched pairs. All tests were performed 2-sided and a P-value <0.05 was deemed statistically significant. All results are presented as median (interquartile range).
RESULTS
A total of 31 women met the criteria for inclusion (Table 1). Median NPi was 28.6 (21.3–40.7). Median NUP was 100.8 (68.3–135.8) mL/hr, whereas median DUP was 132.4 (114.4–160.3) mL/hr (P=0.087).
Thirty subjects met the criteria for GP according to the ≥40 mL/kg/24-hr threshold. Subjects reporting ≥1 nocturnal void(s) (n =30) had a median NPi of 28.5 (21.3–39.4) and a median NUP of 98.8 (68.2–134.1) mL/hr (vs. DUP, 133.1 [115.6–161.3] mL/hr, P =0.058) (Table 2, Fig. 1A). Among these subjects, NPi ≥0.33 and NUP ≥90 mL/hr were 40% and 63% sensitive for GP, respectively.
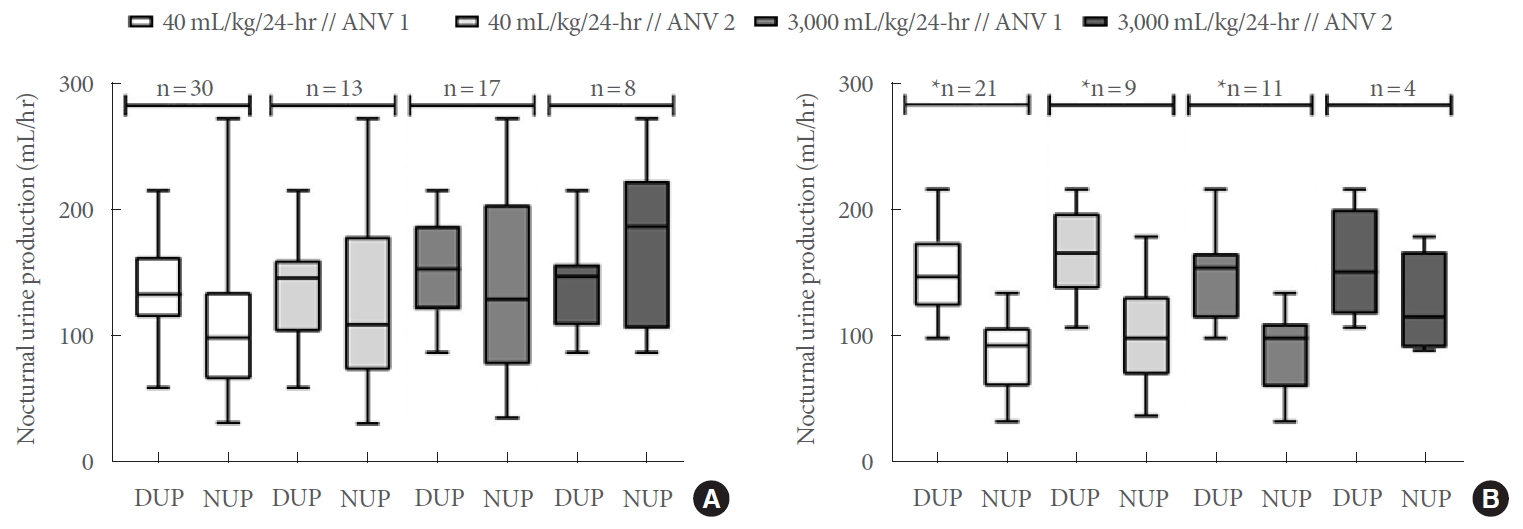
(A) Daytime versus nocturnal urine production in all women. (B) Daytime versus nocturnal urine production in continent women. Horizontal band within boxes denotes median values; box ends reflect 25%–75% interquartile range (IQR); whisker ends reflect minimum and maximum NUP rates among women with global polyuria at each cutoff. ANV, actual number of nocturnal voids (defined as the number of nocturnal awakenings during the intended sleep period; “ANV 1” denotes included subjects with ANV ≥1; “ANV 2” denotes subjects with ANV ≥2); DUP, daytime urine production; NUP, nocturnal urine production. *P<0.05, statistical significance.
The subset of participants reporting ≥2 nocturnal voids (n=17) had a median NPi of 37.7 (22.1–45.0) and a median NUP of 108.9 (86.9–162.1) mL/hr (vs. DUP, 146.0 [109.7 vs. 158.5] mL/hr, P =0.644), wherein NPi ≥0.33 and NUP ≥90 mL/hr were 53% and 71% sensitive for GP, respectively. In a subgroup analysis of subjects without nocturnal urinary incontinence, NPi ≥0.33 and NUP ≥90 mL/hr were 19% and 52% sensitive for GP among participants reporting ≥1 nocturnal void(s) (n=21), respectively, and 27% and 55% sensitive for GP in those with ≥2 nocturnal voids, respectively (n =11) (Fig. 1B).
Thirteen subjects met the criteria for GP at the ≥3000 mL/24-hr cutoff. Subjects reporting ≥1 nocturnal void(s) (n=13) had a median NPi of 28.4 (22.1–42.9) and a median NUP of 128.6 (86.9–196.1) mL/hr (vs. DUP, 153.1 [126.3–182.2] mL/hr, P=0.542). Among these subjects, NPi ≥0.33 and NUP ≥90 mL/hr were 38% and 69% sensitive for GP, respectively.
The subset of participants reporting ≥2 nocturnal voids (n =8) had a median NPi of 42.6 (28.0–46.0) and a median NUP of 187.0 (122.8–215.5) mL/hr (vs. DUP, 146.9 [113.3 vs. 154.5] mL/hr, P =0.461), wherein NPi ≥0.33 and NUP ≥90 mL/hr were 63% and 88% sensitive for GP, respectively. In a subgroup analysis of subjects without nocturnal urinary incontinence, NPi ≥0.33 and NUP ≥90 mL/hr were 11% and 56% sensitive for GP among participants reporting ≥1 nocturnal void(s) (n=9), respectively, and 25% and 75% sensitive for GP in those with ≥2 nocturnal voids, respectively (n=4).
Specific NPi and NUP cutoffs required to capture ≥80%, ≥90%, and 100% of included women with GP are provided in Table 3.
DISCUSSION
The major finding of the present study was that 13%–89% of women with voiding diary-confirmed GP fell below the threshold for a diagnosis of NP, depending on the GP/NP/ANV criteria applied. Suboptimal sensitivity (27%–53%) persisted when the most clinically relevant GP/NP/ANV criteria were employed (GP defined as ≥40 mL/kg/24 hr, in accordance with current International Continence Society terminology, NPi ≥0.33, the most widely recognized NP cutoff, and ANV ≥2, the threshold at which nocturia tends to become clinically significant) [1,12]. Consistently, NUP was significantly or trending lower than DUP across multiple GP/NP/ANV criteria, and the magnitude of this effect increased upon exclusion of subjects with nocturnal incontinence. Taken together, these results suggest that women with GP may produce less urine at night relative to daytime.
This finding has major clinical implications with respect to the diagnostic utility of nocturnal-only voiding diaries, which may be used to screen for nocturia owing to NP or small nocturnal bladder capacity [6]. Real-world use of nocturnal-only diaries is hindered by the fact that a diagnosis of GP is uniformly defined on the basis of 24-hr urine volume [1,7]. Recent research has shown that comorbid GP is characterized by even higher nocturnal urine volumes among men with NP [8], such that low NUP—as is readily ascertainable from a nocturnalonly voiding diary—may be sufficient to rule out underlying GP in men. This finding posits that nocturnal-only diaries offer valuable insight regarding all major broad etiologies of nocturia (i.e., NP, small bladder capacity, and also GP), supporting the rational use of nocturnal-only diaries in the initial evaluation of nocturia in men.
To our knowledge, the present study was the first to recognize that, in contrast to men, some women with GP appear to maintain relatively low nocturnal urine volumes, such that GP may go undetected by a nocturnal-only screening diary. Accordingly, completion of a full 24-hr diary appears to be particularly central to the classification of nocturia in women.
Obstetric and gynecologic factors including parity, pelvic organ prolapse, menopause, estrogen status, and gynecologic surgical history are all directly relevant to nocturia, and thus make nocturia in women a unique entity [13,14]. Much of what is known about the natural history of nocturia has been derived from a large longitudinal population voiding diary study of men, and nocturia in women has been incompletely characterized. While conclusions must be drawn judiciously in view of the modest sample size of the present study, this study was the first, to our knowledge, to specifically characterize a sample of women with nocturia owing to GP.
GP is a rather uncommon entity, and perhaps even more so in women compared to men. GP is present in less than 1-in-5 women with nocturia in some real-world clinical cohorts, even following less stringent cutoffs than those employed in the present analysis [15]. One prior study of voiding diary outcomes in asymptomatic adults reported a median (Interquartile range) 24-hr urine volume of 1,515 (1,100–2,130) mL in women [16]. Assuming these data were normally distributed renders a z-score of 1.70 for a 24-hr urine volume of 2,800 mL (based on the current 40 mL/kg/24-hr International Continence Society cutoff for GP in a 70-kg individual), corresponding to a 1-sided z-critical value of 0.0459, such that 695 women would need to be screened to identify 31 cases of GP. Consistently, a retrospective chart review of women from a single-center urology practice reported a voiding diary completion rate of 30.1%, and an 18.1% prevalence of GP among voiding diary completers, such that requesting voiding diaries from 569 real-world female patients with nocturia would be required to identify 31 women with nocturia owing to voiding diary-confirmed GP [13].
Although GP and NP are both manifestations of excess urine production, current International Continence Society terminology employs a relative weight-based criterion for GP (≥40 mL/kg/24 hr) [1], whereas the most common criteria for NP are either proportion-based (i.e., NPi ≥0.33) or absolute volume-based (i.e., NUP ≥90 mL/hr) [1,11]. Albeit highly dependent on geographic and cultural factors, women tend to weigh less than men [17], which corresponds to a lower absolute volume-per-hour urine production threshold for GP. Interestingly, however, common NP cutoffs had suboptimal sensitivity for detecting GP not only according to the ≥40 mL/kg/24-hr weight-based cutoff [1], but also according to the alternative 3,000 mL/24-hr absolute volume-based cutoff [7]. Therefore, differential rates of NUP among women versus men with GP may not be solely attributable to anthropomorphic differences between the sexes.
Several physiologic mechanisms may contribute to relatively low nocturnal urine volumes amongst women with GP. Plasma concentrations of arginine vasopressin and other neuroendocrine signaling peptides responsible for water and solute equilibrium follow a well-defined circadian rhythm such that the nocturnal period is characterized by a marked decrease in the excretion of water and several major electrolytes [18]. In animal models, female vs. male rats exhibited significantly greater renal expression of vasopressin 2 receptor (V2R) and greater physiologic responses to V2R agonism [19]. In humans, women tend to be more sensitive to the effects of arginine vasopressin replacement therapy [20]. Consequently, both differential secretion and response to endogenous arginine vasopressin may contribute to differential nocturnal urine volumes and circadian rhythmicity in urine production by sex. Additional contributory mechanisms may include female reproductive hormones, which are associated with changes in homeostatic set points for fluid regulation, as coadministration of progesterone and estradiol has been found to increase both extracellular and plasma volumes [21].
One notable limitation of the present analysis was the absence of data pertaining to dietary and fluid intake. Although undoubtedly highly variable across populations, men tend to consume greater quantities of free water and dietary protein (with the former strongly associated with urine volume, and the latter known to acutely augment glomerular filtration rate) [22-25]. It stands to reason that evening dietary intake may also contribute to sex differences in NUP. Increased 24-hr urine production with a strong predilection for the hours awake may also be suggestive of primary polydipsia, although the prevalence of primary polydipsia in community-dwelling women and men without a prerequisite mental health diagnosis remains unknown [26]. Likewise, data from the National Health and Nutrition Examination Survey suggest that adult and elderly males in the United States consume upwards of 1 g more dietary sodium compared to their female contemporaries [27], and high dietary sodium is associated with both greater NPi and NUP [28].
The present study is also subject to the inherent limitations of a single institution analysis with a limited number of participants. The duration and severity of nocturia and other lower urinary tract symptoms could not be assessed. Additionally, comorbidities associated with increased urine production, such as obstructive sleep apnea, cardiovascular disease, diabetes mellitus, diabetes insipidus, and metabolic syndrome, were not taken into account. Similarly, data pertaining to the use of diuretics and other medications were not available, which may have had bearing on the observed trends as well as serum sodium levels. Larger study samples with well-defined GP patient subgroups are needed to establish reproducible nocturnal urine cutoff values and further elucidate the relationship between nighttime and DUP in both women and men with GP. It stands to reason that nocturia-specific and global lower urinary tract symptom questionnaires, which are routinely used as adjunctive instruments in the clinical management of nocturia, would also be highly useful in characterizing the population of nocturia patients with GP. Future research must also concurrently employ intake diaries to account for the possibility of primary polydipsia. Notwithstanding these limitations, the present findings underscore the need for greater representation of women in nocturia literature.
In conclusion, GP is one of the major pathophysiologic mechanisms of nocturia and a harbinger of serious systemic disease [2]. Recent research suggests that low NUP may be sufficient to rule out underlying GP in men. However, the present analysis demonstrates that women with GP may instead produce relatively little urine at night, such that indices of nocturnal urination are fairly insensitive for GP. Accordingly, completion of a full 24-hr diary thus remains particularly important in classifying the etiology of nocturia in women as to not miss underlying GP, underscoring the need for future research on the pathophysiology of GP and clinical predictors of a daytime predominance in urinary output in these patients. Potential sex differences in the pathophysiology of GP also merit further investigation.
Notes
Research Ethics
Ethical approval was obtained from the Ghent University Hospital ethics committee (EC/2011/565; EC/2013/950) and all subjects provided written informed consent in accordance with the Declaration of Helsinki.
Conflict of Interest
Thomas F. Monaghan has no direct or indirect commercial incentive associated with publishing this article and certifies that all conflicts of interest relevant to the subject matter discussed in the manuscript are the following: Donald L. Bliwise has served as a consultant for Merck, Jazz, Ferring, Eisai, and Respicardia and speaker for Merck within the last 3 years, outside the submitted work. Lori A. Birder is a consultant for Ferring, Urogen, and Allergan, outside the submitted work. Johan Vande Walle reports institutional grants from Allergan, Astellas, and Ferring, and is a consultant and lecturer for Ferring and Astellas, outside the submitted work. Alan J. Wein has served as an advisor/consultant for Bulkamid, Medtronic, Serenity, Urovant, and Velicept, outside the submitted work. Jerry G. Blaivas is co-founder and chief-scientific officer of Symptelligence Medical Informatics, LLC, outside the submitted work. Jeffrey P. Weiss is a consultant for Ferring, and the Institute for Bladder and Prostate Research, outside the submitted work. Karel Everaert is a consultant and lecturer for Medtronic and Ferring and reports institutional grants from Allergan, Ferring, Astellas, and Medtronic, outside the submitted work. The other authors have nothing to disclose.
AUTHOR CONTRIBUTION STATEMENT
·Conceptualization: TFM, KE
·Data curation: TFM, AMK, CWA, JML, USA, JVW, KE
·Formal analysis: TFM, AMK, JML, JPW, KE
·Methodology: TFM
·Project administration: KE
·Visualization: TFM
·Writing-original draft: TFM
·Writing-review & editing: TFM, AMK, CWA, SNR, KPM, DLB, JML, LAB, USA, JVW, AJW, JGB, JPW, KE