Correlation Between Testosterone Replacement Treatment and Lower Urinary Tract Symptoms
Article information
Abstract
Lower urinary tract symptoms (LUTS) are a cluster of voiding symptoms, such as weak stream, hesitancy, intermittency, urinary frequency, urgency, and nocturia. LUTS are frequent in elderly men and it considered the ultimate clinical symptoms of benign prostatic hyperplasia. With aging, male hypogonadism is increased which is defined as decreased ability of the testes to produce sperm and sex steroids because of a pituitary/hypothalamic, or testicular deficiency. In academic andrology associations, the term “male hypogonadism” is commonly used to categorize testosterone deficiency. Testosterone deficiency syndrome (TDS) is defined as a decrease in serum testosterone accompanied by symptoms such as libido decrease, depressive disorder, erectile dysfunction, and fatigue. Although the mechanism about testosterone-replacement therapy (TRT) effects on men with hypogonadism is not yet identified, TRT has been shown to effectively relieve the symptoms of TDS as well as LUTS by several studies. Although the present review demonstrates the effectiveness and safety of TRT in men with TDS by prior studies, future large scale of clinical trials should be conducted to present more high-quality evidence to clinicians and patients.
INTRODUCTION
Lower urinary tract symptoms (LUTS) are a cluster of voiding symptoms, such as weak stream, hesitancy, intermittency, urinary frequency, urgency, and nocturia. LUTS are considered the ultimate clinical manifestation of benign prostatic hyperplasia (BPH) and benign prostatic enlargement, which cause bladder outlet obstruction [1,2]. With the increasing population of aging males, many recent studies have focused on extending life and prolonging good health in these individuals [1,3]. In this regard, many researchers have investigated testosterone, which is related to the differentiation and development of male genital organs.
Testosterone deficiency syndrome (TDS) is defined as a decrease in serum testosterone accompanied by symptoms such as libido decrease, depressive disorder, erectile dysfunction, and fatigue [4]. Several studies have reported that men experience a decline in testosterone levels beginning in their 40s, and that about 30% of men meet the TDS criteria at the age of 70 years [5-7]. In the Boston Area Community Health Survey, the prevalence of symptomatic TDS among men aged 30–79 years was about 6% [8]. In addition, 26.5% of men in their 60s and 70s have reported bothersome LUTS [9].
Testosterone replacement therapy (TRT) has been shown to effectively relieve the symptoms of TDS [10-16]. However, the increasing use of TRT among men who are at risk for LUTS has raised concerns about its potential adverse effects [17]. In particular, TRT may exacerbate LUTS because androgens play an integral role in the development and growth of the prostate epithelium [18].
Longitudinal studies have not consistently associated testosterone with the development of LUTS after adjusting for age [19]. However, Favilla et al. reported that high levels of testosterone were associated with an increased risk of severe LUTS [18], which have been listed by the Endocrine Society as a relative contraindication to the TRT because the treatment may worsen symptoms [20]. Some studies have demonstrated that TRT does not aggravate LUTS among men with TDS, but their results have not yet been formally accepted [14,21-25]. Relatedly, several of the Endocrine Society’s recommendations pertaining to TRT have recently been critically reevaluated [26].
Thus, in the present study, we performed a systematic review of the relationship between TRT and LUTS to clarify the available evidence.
METHODS
We searched relevant words in PubMed, Embase, and the Cochrane Library. The researched terms were as follows: “benign prostatic hyperplasia” AND “testosterone” AND “Hypogonadism” OR “International Prostate Symptom Score” OR “prostate volume” OR “safety” OR “adverse events”. The present query was limited to retrospective or prospective human trials published in English. There were no limitations on publication date or follow-up period.
TESTES, PROSTATE, AND ANDROGENS
The testes are involved in spermatogenesis, which requires the seminiferous tubules to be intact and functioning. In addition, it has a steroidogenic function, secreting steroid sex hormones from the Leydig cells [27]. Spermatogenesis is a highly complex process involving subtle and continuous interactions between paracrine and autocrine regulators, of which testosterone is the most important [28]. The prostate weighs about 1–2 g at birth; in early puberty, it increases to approximately 10 g and finally to an average of 20 g in young adults [29-31]. After this phase, in which proliferation occurs in all 3 zones of the prostate gland (peripheral, central, and transitional zones [TZs]), approximately half of men show a second, selective growth phase in the TZ by their 50s, while about 90% of men older than 80 years have undergone this second phase.
Prostate Development and Androgen Levels
During early fetal life, the prostate tissue differentiates via branching morphogenesis, which involves the organization of epithelial buds from the urogenital sinus into the surrounding mesenchymal tissue [32]. Studies using recombinant tissues composed of wild-type epithelium and androgen receptor (AR)-deficient mesenchyme have shown that the action of androgens on mesenchymal cells is necessary for prostate differentiation [33,34]. The ventral mesenchymal pad forms in a similar way in both male and female rats, but a normal prostate only develops when female rats are administered androgens [35], which also suggests that androgens play a role in early prostate development. The growth factor andromedin is androgen-dependent, but it is not clear whether AR action directly stimulates its secretion directly or rather whether androgens are indirectly involved in its activity or availability [32]. It is likely that AR only acts on epithelial cells at a later stage of differentiation, when it is necessary for the cells’ exocrine secretory function and for their differentiation from basal to terminal luminal cells [36].
Prostate Growth and Androgens
Besides prostate development, androgens play an important role in prostate proliferation. Approximately 90% of prostatic androgens are in the form of dihydrotestosterone (DHT), which is mainly derived from the testis; only 10% comprise androgens of adrenal origin. The enzyme 5α-reductase converts testosterone to DHT, which is 5 times more potent than testosterone. In prostatic cells, DHT binds to the AR with high affinity [37-39].
In male fetuses, testosterone levels begin to increase in the 8th week of gestation and peak at the 16th week, when the concentration of testosterone is similar to that of adults. The hormone then declines to levels similar to those at birth.
Prostate growth occurs in 3 phases [32]. The first occurs during fetal life and ends at birth. After birth, testosterone increases again for 6 months—known as “mini-puberty”—and then decreases to undetectable levels during childhood [32,40]. With the decline in testosterone after mini-puberty, prostate volume decreases and its growth is negligible until puberty [41], when the second phase of growth occurs in response to the increase in testosterone. At this stage, cell development and differentiation occur, leading to the adult prostate. The third and last phase unfolds in middle-age and continues throughout adolescence. The prostate is unlike other organs in that it continues to grow throughout adulthood. However, the third phase corresponds to a decline in testosterone levels [42]. In this regard, while the development of the prostate during the first 2 phases is clearly androgen-dependent, the role of androgens in the third phase is still unclear [43].
Testosterone Decline and BPH
With aging, the incidence of BPH increases, and testosterone levels reduce because of impaired testicular function [44,45]. Specifically, testosterone decreases by about 2% annually in men [6]. The prevalence of TDS has ranged from 6% to 38% in several studies [8,46]. In previous studies, no significant relationship has been found between testosterone and prostate size [37-39], while in others, androgen replacement therapy was correlated with an improvement in LUTS [43,47]. These observations suggest that androgens are not directly related to the development of BPH.
Conversely, several experimental and clinical studies have shown results that are inconsistent with this conclusion. For example, TRT causes BPH in young castrated dogs, and no one who underwent simple orchiectomy during childhood develops BPH [38]. Similarly, those with low levels of 5α-reductase type 2 show a relatively small prostate [48]. In Korean men with prostate cancer, androgen deprivation therapy (ADT) caused a decrease in prostate volume, improved urodynamic parameters, and alleviated LUTS [49]. Administration of 5α-reductase inhibitor decreases prostate size [50]. To explain these results, some authors have hypothesized that prostatic hyperplasia is related to intraprostatic concentrations of DHT rather than to circulating free testosterone levels [39].
However, in a small, randomized control trial there was no evidence of increased intraprostatic DHT in patients given TRT [23]. Favilla et al. reported no significant association between BPH and testosterone among 122 men with BPH, even though LUTS is associated with testosterone levels [18].
BPH/LUTS, Prostatic Inflammation, and Androgens
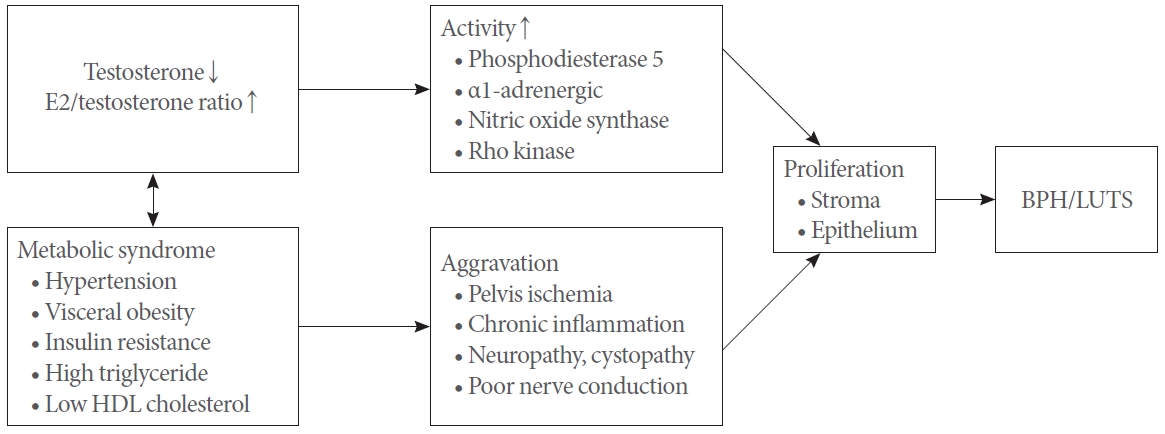
The prostate is an organized, immunocompetent tissue that contains several immune cells, including lymphocytes, granulocytes, and macrophages. In this context, such cells are termed prostate-associated lymphoid tissue, which when activated can initiate a chronic immune response that persists even when primary inflammatory stimulants subsided. T helper I lymphocytes secrete interferon (IFN)- and interleukin (IL)-2, which are the main cytokines found in the early phase of BPH [51]. Lymphocytes and macrophages produce IFN-c and IL-17, which stimulate the production of chemokines by stromal cells. These interactions appear to induce prostate cell proliferation and BPH development [52,53]. The increase in IL-17 has been related to increased secretion by prostate stromal cells of IL-6 and IL-8, which are important factors in prostate hyperplasia [54]. Many clinical studies have reported that patients with prostatitis are at greater risk for BPH/LUTS. Thus, inflammation may lead to BPH development of [55,56]. Despite these findings, it is unclear whether any preexisting conditions cause BPH.
Relatedly, Vignozzi et al. evaluated prostate inflammation and tissue remodeling accompanied by decreased testosterone levels and high estrogen levels in male rabbits with metabolic syndrome (MetS). The authors suggested that hypogonadism plays a critical role in the development of prostate inflammation [39,43]. Together with other results, this indicates that, in patients with both hypogonadism and prostate inflammation, TRT may alleviate inflammatory changes and prevent the development of BPH or LUTS [3,39,57,58]. Fig. 1 shows these interactions schematically.
BPH/LUTS and MetS
MetS is a group of metabolic diseases that share insulin resistance as their main pathogenic mechanism. It is known as a risk factor for BPH development [3]. However, the mechanisms by which this occurs are highly complex; hyperinsulinism is associated with stimulation of the insulin-like growth factor (IGF)-1 receptor, higher IGF-1 levels, lower IGF-1 binding, and higher free calcium in smooth muscle and neural cells. In addition, it activates the sympathetic nervous system and increases the tone of prostatic smooth muscle [58]. Therefore, insulin-sensitizing drugs are recommended to treat MetS [59,60].
In a large British study, the risk of MetS was 37% higher in men with BPH than in those without [32]. A review of 8 studies including a total of 5,403 men with MetS showed a significantly larger prostate and a TZ volume that was 4 mL greater [61]. One study involved 171 men with a mean age of 36.5±8.3 years who had visited the urology clinic for counsel about infertility. The results showed that the severity of MetS was associated with a larger prostate volume [62]. In the Prostate Cancer Prevention Trial, new occurrence of BPH over a 10-year follow-up was predicted by higher serum C-reactive protein and IL-6 levels at baseline [63], suggesting that conditions characterized by systemic inflammation, such as MetS, might be implicated in BPH development [58,64-66].
MALE HYPOGONADISM
Male hypogonadism is defined as decreased ability of the testes to produce sperm and sex steroids because of a pituitary/hypothalamic, or testicular deficiency [27,67]. The latter is known as primary hypogonadism, while the former is termed secondary or central hypogonadism. In academic andrology associations, the term “male hypogonadism” is commonly used to categorize testosterone deficiency, which is associated with infertility.
Definition and Diagnosis of TDS
When low testosterone is related to age and/or comorbidity (e.g., hypertension, coronary vascular disease, obesity, dyslipidemia, impaired insulin sensitivity) and is associated with symptoms (e.g., decreased libido, erectile dysfunction, fatigue, weakness) and signs (e.g., increased abdominal fat, reduced muscle and bone mass, gynecomastia, and small testes), it can be defined as TDS [11,67], which is a mixed type of hypogonadism involving both central and peripheral components [16].
Laboratory measurements should be performed in the morning or within 2 hours of getting up because testosterone levels show circadian variations [68]. In many laboratories, the lower normal limit is between 250 and 350 ng/dL (8.7–12.2 nmol/L). However, if patients with borderline levels of testosterone, their free testosterone or sex hormone-binding globulin should be measured [67-69]. According to other guidelines, TRT is recommended to subjects with levels <8 nmol/L (231 ng/dL), while those with level >12 nmol/L (346 ng/dL) do not require TRT. If the patient’s testosterone level is between 8 and 12 nmol/L and they show hypogonadal symptoms (mentioned above), TRT might be seriously considered [67,69,70]. Additionally, Wu et al. [71] newly defined TDS based on data from the EMAS study. They mentioned a syndromic association between low testosterone levels (<11 nmol/L) and sexual symptoms, namely decreased libido and erectile dysfunction [71].
TRT ON TDS
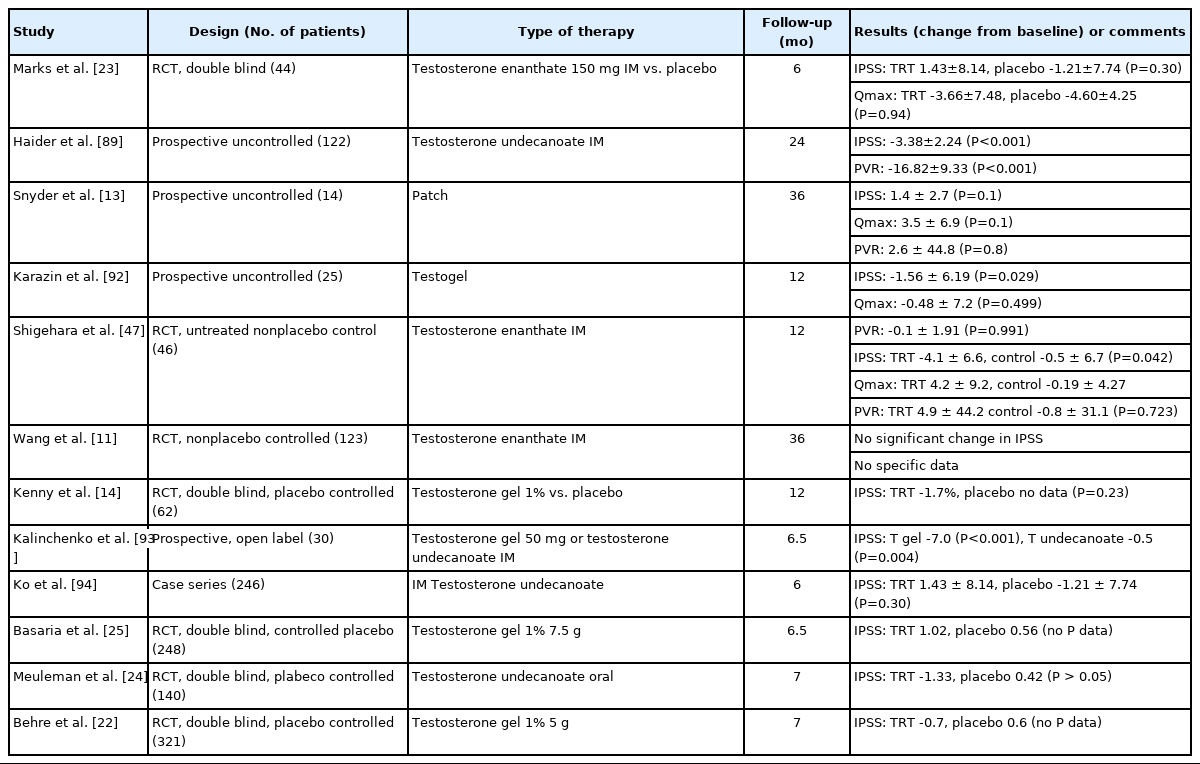
Patients with TDS should be evaluated individually to ensure that clinicians select the best-matched treatment. The types of TRT are presented in Table 1. No treatment guidelines are available for TDS, although if the low testosterone levels are related to any other comorbidities, it should be corrected at first [16]. However, TRT is the only treatment choice in cases of primary hypogonadism. According to the 2015 cardiovascular risk advisory, the Food and Drug Administration approved TRT specifically to treat men with low testosterone levels caused by disorders of the testes, brain, or pituitary gland that can cause hypogonadism, but not to treat men who only showed age-related low testosterone [25]. Finally, clinicians should consider whether the benefits of TRT overcome the potential risks. Currently, different types and methods of TRT are available.
General Thinking About TRT and BPH/LUTS
In the past, clinicians rose several concerns about the risk of BPH/LUTS and acute urinary retention after TRT, based on a report by Huggins and Hodges in the 1940s, who studied a small number of patients with metastatic prostate cancer and found that castration resulted in disease regression and that only one patient who had undergone TRT showed cancer extension [72]. Holmäng et al. [73] reported that prostate volume may increase by approximately 12% with TRT. However, observational studies have failed to show that testosterone levels are significantly correlated with BPH, and clinical trials have found no definite correlation with serum prostate-specific antigen (PSA) or prostate volume in patients with normal testosterone levels [74]. Previous major urological guidelines have reflected these concerns [67,75]. However, the current TRT guidelines of the European Association of Urology no longer regard severe LUTS (International Prostate Symptom Score [IPSS] >19) as an absolute contraindication, and regarded as a relative contraindication [76]. The guidelines also note that this paradox is not yet supported by strong clinical evidence, and that, if LUTS is treated appropriately, it is no longer a definite contraindication to TRT.
Furthermore, TRT may improve nocturia, which is the most bothersome LUTS in men with TDS. Testosterone levels may be correlated with circadian rhythms and sleep quality, which are perturbed by nocturia [77]. Indeed, several studies have reported that TDS has a negative effect on overall sleep quality, and that this can be attenuated by TRT [77].
Conversely, with regards to reducing testosterone levels in patients with LUTS, Washino et al. [78] reported that ADT significantly reduced prostate size in patients with prostate cancer, but increased nocturia in patients with mild LUTS. The authors reasoned that ADT suppresses serum levels of testosterone in older men, which may disrupt the urine concentrating function of the kidneys by decreasing circulating vasopressin and vasopressin binding sites, resulting in the development of nocturnal polyuria. Although controversies exist, TRT adaptation has rapidly increased, especially in males with TDS [79].
Evidence for the Benefits of TRT in BPH/LUTS
Prior to the present evidence, we reviewed previous studies, which are presented in Table 2. Recent studies have reported that TRT does not worsen LUTS, but rather improves it. Indeed, several studies have shown that testosterone plays a significant role in bladder and urinary sphincter function.
Similar to human clinical trials, animal studies have shown that TRT can improve LUTS. Tek et al. [80] reported that TRT improves LUTS and bladder function, showing increased bladder capacity and compliance, as well as decreased detrusor pressure at maximum flow rate (Qmax). In addition, Celayir [81] evaluated the relationship between bladder function and testosterone levels in castrated rabbits with or without TRT. Bladder capacity and compliance improved after 5 and 10 days in the TRT subjects, but decreased thereafter and returned to baseline levels after 30 days. This suggests that TRT is related to bladder capacity and compliance. In another rabbit study, ARs were found in the pelvic autonomic nervous system, detrusor muscle, bladder mucosa, striated sphincter, and urethral mucosa. In the case of rats, abundant ARs were found in the pelvic autonomic nervous system [82].
Wilt et al. [83] investigated prostate cancer treatment outcomes and reported that urinary incontinence (≥1 time/day) occurred in 35% of men after radical prostatectomy and in 12% of men after radiotherapy. Surprisingly, among those who were only treated using ADT, 11% complained of urinary incontinence, perhaps because of external aging-related degenerative changes in the urinary striated sphincter. Testosterone levels are associated with muscle quality and quantity [84], which might be why a decline in testosterone levels stimulates apoptosis [85]. As early as 1938, Walther and Willoughby [86] used TRT over 2 years in 15 men with BPH/LUTS, but their study was overlooked or neglected for some period. Pearl et al. [87] found no significant relationship between TRT and IPSS in 120 men in a 1-year follow-up study. Interestingly, in many men treated using TRT, IPSS scores were decreased by more than 3 points. Yassin et al. [88] performed a prospective observational study to evaluate the effects of TRT on LUTS in 261 men with TDS. The mean IPSS significantly improved from 10.35 to 6.58 during a median follow-up of 42.3 months. Shigehara et al. [47] performed a randomized trial for 1 year among 52 men with BPH/LUTS. They reported that post-TRT IPSS (12.5±9.5) was significantly improved compared with baseline IPSS (15.7 ± 8.7), and that Qmax was also improved, while postvoid residual (PVR) was not. Haider et al. [89] treated 117 men with TDS (mean age, 59.5±6.0 years) using injectable testosterone undecanoate (1,000 mg) for 3 months. The results showed LUTS improvement. Both IPSS and PVR decreased, but no change was observed in total prostate volume. One multinational, multicenter study involved 999 men with both TDS and prostate cancer (mean age, 59.1±10.5 years) who were followed up for a median of 3 years. A total of 75% had TRT, while 25% had no TRT. The TRT group showed LUTS improvement compared with the untreated group [90]. Kohn et al. [91] performed a systematic meta-analysis of 14 trials involving a total of 2,029 men to evaluate whether TRT indeed exacerbates LUTS. There were no significant differences in IPSS changes from baseline between the TRT and placebo treatment groups. The authors concluded that TRT does not worsen LUTS in men with TDS.
In addition, nocturia is prevalent among elderly men with LUTS and is one of the most serious symptoms. Shigehara et al. [77] reviewed the effects of TRT and nocturia. Testosterone activates endothelial nitric oxide synthase in the pelvis, increasing nitric oxide concentration in the blood vessel tissues. This may result in the dilatation of pelvic blood vessels and relief of pelvic ischemic change. Previous studies have reported that TRT for 1 year in men with TDS conferred increased maximal bladder capacity and bladder compliance, and that it decreased detrusor pressure at Qmax [47,87,93,94]. Therefore, there is enough evidence to treat men with TDS without unnecessary concerns about LUTS. However, there is still a lack of large, double-blind, randomized clinical trials, more clinical studies should be conducted to identify the exact beneficial effects of testosterone.
Evidence for TRT Risk in BPH/LUTS
Historically, clinicians have been concerned that TRT increases LUTS, but the present study found no evidence of significant exacerbation of LUTS after TRT, although many studies have excluded subjects with severe LUTS at baseline [91].
Pearl et al. [87] retrospectively studied 120 men with TDS who underwent TRT and reported that the treatment carries a low risk of exacerbating LUTS and that the changes in PSA levels were minor.
Several studies have stated that TRT in older men with TDS alters PSA levels, but that it rarely increases over the normal upper level. However, TDS constitutes a relative contraindication to TRT, according to several guidelines [67,69,76].
CONCLUSIONS
The present review showed no evidence to support warnings that TRT worsens LUTS in men with TDS. TRT is already used worldwide as a treatment for TDS, and much evidence is still emerging regarding this treatment. Furthermore, TRT may also improve nocturia in men with TDS. Therefore, it is enough to warn patients that LUTS may worsen after TRT. However, further high-quality evidence is needed to inform clinicians using TRT to treat men with both TDS and severe LUTS.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTION STATEMENT
·Conceptualization: SCK
·Data curation: MHL, YSS, SCK
·Formal analysis: MHL
·Methodology: MHL
·Project administration: SCK
·Visualization: MHL
·Writing-original draft: MHL
·Writing-review & editing: YSS, SCK