Endoscopic Botulinum Toxin Injection for Refractory Enuresis Based on Urodynamic Assessment
Article information
Abstract
Purpose
This study aimed to determine the urodynamic characteristics of refractory enuresis and explored whether those characteristics can be managed through differential endoscopic injections with botulinum toxin.
Methods
In total, 27 patients with nonmonosymptomatic enuresis who showed no response after conservative treatment for more than 12 months were included. The patients then underwent a videourodynamic study and received a differential endoscopic injection of botulinum toxin on the same day. Reduced capacity, detrusor overactivity, and bladder neck widening were the 3 major abnormal findings assessed during the filling phase, while sphincter hyperactivity was the only abnormality assessed during the emptying phase. An intravesical or intrasphincteric injection of botulinum toxin was attempted according to the videourodynamic study findings. Follow-up was conducted at 1, 3, 6, and 12 months after treatment.
Results
The median age was 10 years (range, 7–31 years). Although 19 and 8 patients had a preoperative diagnosis of overactive bladder or dysfunctional voiding, respectively, the urodynamic diagnosis was different in more than half of the patients. Those showing detrusor overactivity benefited from intravesical botulinum toxin injection, whereas those with only sphincter hyperactivity benefited from both intravesical and intrasphincteric injections. Treatment resistance to botulinum toxin seemed to be attributable to bladder neck widening. Time had no apparent effect on efficacy, which persisted 6 months after the injection. More than 80% of the patients maintained the benefits of the injection after 1 year.
Conclusions
Videourodynamic studies were useful for identifying the reasons underlying refractory nonmonosymptomatic enuresis and helpful for determining the appropriate site of botulinum toxin injection.
INTRODUCTION
Enuresis is a common problem that can be quite bothersome. Although most cases resolve spontaneously with age, some patients experience a protracted course and even persist into adulthood [1]. Cases that persist into adulthood may cause significant distress in affected individuals, potentially leading to social withdrawal [2]. Given the significant individual variation in the natural resolution of this problem, it is inappropriate to withhold treatment on the premise that children will eventually experience spontaneous resolution, especially in severe cases wherein spontaneous resolution is unlikely [1,3].
Once the decision is made to treat enuresis, the first step involves determining whether patients experience daytime symptoms caused by lower urinary tract dysfunction (LUTD) [3,4]. Accordingly, enuresis can be categorized as monosymptomatic enuresis (ME) or nonmonosymptomatic enuresis (NME) based on the presence of LUTD. The treatment for NME is complicated by the presence of multiple relevant etiologies that need to be addressed individually in accordance with the pathophysiology. A study of patients with nonneurogenic voiding dysfunction revealed that most LUTD found in NME cases could be classified into either overactive bladder (OAB) or dysfunctional voiding (DV) [5]. Since both are urodynamic abnormalities, the differential diagnosis between the 2 etiologies should, in principle, require an invasive urodynamic study (UDS). In practice, however, the diagnosis is made with noninvasive diagnostics, including symptomatology, a voiding diary, and uroflowmetry with or without simultaneous electromyography (EMG). Fortunately, management based on noninvasive diagnostics works well in most cases. After successfully converting NME into ME through appropriate treatment, the latter could then be managed using established treatment. Occasionally, however, cases refractory to treatment emerge and often need invasive diagnostics to identify new and relevant pathologic findings that could guide treatment. Cystometry in these patients may reveal novel pathophysiology previously undetectable with noninvasive diagnostics [6]. The addition of fluoroscopy (videourodynamic study [VUDS]) or needle EMG may facilitate the correct identification of sphincter movement [7]. Potential urodynamic pathologies include detrusor overactivity (DO), sphincter hyperactivity (SH) during voiding (dysfunctional voiding), bladder outlet obstruction (or bladder neck dysfunction), and intrinsic deficiency of sphincter function, as previously reported [5,6,8]. Although VUDS can reveal the potential contribution of the aforementioned etiologies, the likelihood of identifying relevant findings has been reported to be low [9].
The reported 60%–80% efficacy of drugs and biofeedback for the management of OAB and DV [10] implies that approximately 20%–40% of cases do not respond to treatment. The odds of controlling enuresis therefore remain unacceptable, which constitutes another reason why managing NME is difficult.
Botulinum toxin (BTX) has been applied to treat refractory OAB and DV in both children and adults. Since most cases of refractory enuresis were found to have either OAB or DV, BTX injections may be effective in treating enuresis and could facilitate the control of LUTD [11,12]. Thus, we hypothesized that VUDS would reveal the underlying LUTD in patients with refractory enuresis, which would be effectively addressed by selective BTX injection. Our treatment was designed such that it could be completed within 1 day, because most parents expressed reluctance to have the diagnostic procedure and injection treatment separated due to the resulting frequent absences from school. Instead, they preferred to complete the diagnostic VUDS and therapeutic BTX injection in a single day.
The current study details our experiences with endoscopic treatment of children with refractory enuresis. To identify the appropriate injection site, VUDS was performed beforehand. This study aimed to determine the underlying urodynamic problems among patients with refractory enuresis and to investigate the treatment course following endoscopic injection.
MATERIALS AND METHODS
This is retrospective evaluation of a case series conducted by reviewing our database of patients with enuresis. This study was approved by the Institutional Review Board (IRB) of Seoul National University Hospital Biomedical Research Institute (IRB No.1509-093-705).
Management Protocol and Determination of Treatment Refractoriness
Patients with enuresis were initially evaluated with the Dysfunctional Voiding Symptoms Score (DVSS), a selective physical examination of the back and genitalia, and a 2-day voiding diary. Those without LUTD in DVSS were offered either desmopressin or an enuretic alarm, while those with LUTD were requested to undergo uroflowmetry with simultaneous EMG and postvoid residual (PVR) urine measurement. Those who were diagnosed with NME were further classified into OAB and DV groups based on their clinical features and noninvasive assessment. Those with OAB featured frequency and urgency in their history and a small voided volume in their voiding diary. Uroflowmetry showed tower- or bell-shaped curve patterns and minimal PVR. Those with DV were mainly characterized by staccato or intermittent uroflowmetry. They often showed features of emptying symptoms, increased PVR, or significant fecal impaction.
To address LUTD, patients initially received urotherapy with regular water intake and timed voiding and an active bowel control regimen for 2 months. Then, those who did not show improvement received an antimuscarinic agent (solifenacin, 5 mg/day) or biofeedback with pelvic floor muscle training for 3 or 4 months. Patients without improvements in the DVSS required further treatment. For the next 6 months, those with OAB received double antimuscarinics (solifenacin [5–10 mg/day] and oxybutynin [10 mg/day]) and biofeedback for the first and the last 3 months, respectively. Meanwhile, those with DV received biofeedback and antimuscarinics for the first 3 months and an alpha-antagonist and antimuscarinics for the last 3 months.
Refractory enuresis was confirmed when no improvement was observed after 1 year of various treatments in the presence of good treatment compliance. Such patients were considered potential candidates for salvage treatment using BTX. Since most parents were eager to obtain control of enuresis as early as possible, they agreed to the treatment and provided informed consent.
VUDS and Endoscopic Injection of BTX
On the day of treatment, patients were admitted to the outpatient surgery center and transferred to the operating room where VUDS and the subsequent injection were performed. VUDS was conducted according to the International Children’s Continence Society (ICCS) standards [13,14]. Accordingly, 1 or 2 study cycles were performed depending on whether clinically relevant findings were detected. After placing patients in the supine position, a double-lumen 6F cystometry catheter and an 8F rectal balloon catheter were inserted. The bladder was filled with sterile normal saline mixed with a contrast medium at 10% of the expected bladder capacity. Patients were allowed to void upon feeling the urge before capacity was reached. When the age-adjusted expected capacity was reached, patients were asked to void in either the supine or sitting position and were observed for 20 minutes for spontaneous voiding. A sense of bladder filling, cystometric capacity, DO, bladder compliance, and widening of the bladder neck (WBN) were assessed during the filling phase of VUDS. SH during the voiding phase was defined as electromyographic hyperactivity or a shuttering sphincter movement that resembled a spinning top on fluoroscopy during spontaneous voiding. Patients who failed to void in the presence of the catheter for more than 20 minutes were assumed to have SH. Following VUDS and determination of the etiology of enuresis, patients were placed in the lithotomy position and general anesthesia was established. During this preparation, treatment was planned based on the VUDS findings.
Further injection procedures were attempted to correct abnormalities revealed during the VUDS. Intravesical BTX was attempted when findings suggested reduced capacity (RC) or DO. BTX was diluted in normal saline at 10 U/kg to a maximum of 300 U. Multiple injections were distributed throughout the detrusor at up to 20 sites. Patients suspected of having SH received intrasphincteric BTX diluted in normal saline to 25–33 U/mL and injected into the external urethral sphincter across 3 to 4 quadrants to a maximum of 100 U [15,16]. After the injection, patients were observed for 4 hours to detect potential complications, after which they were discharged home.
Follow-up and Assessment of Treatment Response
Patients were advised to continue with standard urotherapy and were followed up 1 month after the injection. When improved responses were not observed at the 1-month follow-up, desmopressin or the prior anticholinergics were added to enhance responses. Changes in enuresis and lower urinary tract symptoms were assessed every 3 months for 1 year. Responses were evaluated according to the ICCS criteria [17].
RESULTS
Since 2016, among the 282 patients with enuresis who were treated with the same treatment protocol at Seoul National University Children’s Hospital, 27 (10.4%; 14 boys and 13 girls) ultimately received endoscopic treatment (Table 1). All patients were considered to have NME and exhibited at least 1 kind of storage (urgency and frequency) or emptying (straining and intermittency) symptom. More than three-quarters of them (21 patients) still showed concomitant daytime incontinence. A history of constipation was noted in 13 patients (48%), who received polyethylene glycol treatment for a median of 7 months (range, 4–31 months). Based on their clinical findings, 19 and 8 patients were diagnosed with OAB and DV, respectively. Prior to endoscopic treatment, patients received a median of 16 months (range, 6–31) of treatment for enuresis and LUTD, as described previously. Overall, 7 (36.8%) and 3 patients (37.5%) with OAB and DV exhibited improvement in their daytime incontinence or daytime symptoms, although no patient showed improvements of enuresis. With regard to the voiding diary, none of the patients with OAB and only 2 patients with DV showed normalized maximal and average voided volumes. Thus, both were determined to be treatment-resistant.

Clinical features of the 27 patients who underwent endoscopic injections following videourodynamic studies
Fig. 1 presents the VUDS results for the included patients. The 2 major abnormalities observed during the filling phase were DO and RC, which were found in 14 (52%) and 22 patients (82%), respectively. During the voiding phase, 15 patients (56%) had SH. Additionally, 9 patients (33%) showed gradual WBN during the filling phase. Among the 19 patients clinically presumed to have OAB, 11 (58%) and 16 (84%) had DO and RC, respectively. Four patients (21%) showed WBN during the filling phase. During the voiding phase, 9 patients (47%) showed synergic voiding. Among the 8 patients presumed to have DV, 3 (38%) and 6 (75%) had DO and RC, respectively. During the filling phase, 5 (63%) showed WBN, while during the voiding phase, the same number of patients showed SH. Four patients presumed to have OAB failed to void despite being catheterized. They were subsequently diagnosed with SH and grouped with the 6 patients who showed RC but not DO. Nondilating vesicoureteral reflux was observed in 2 patients.
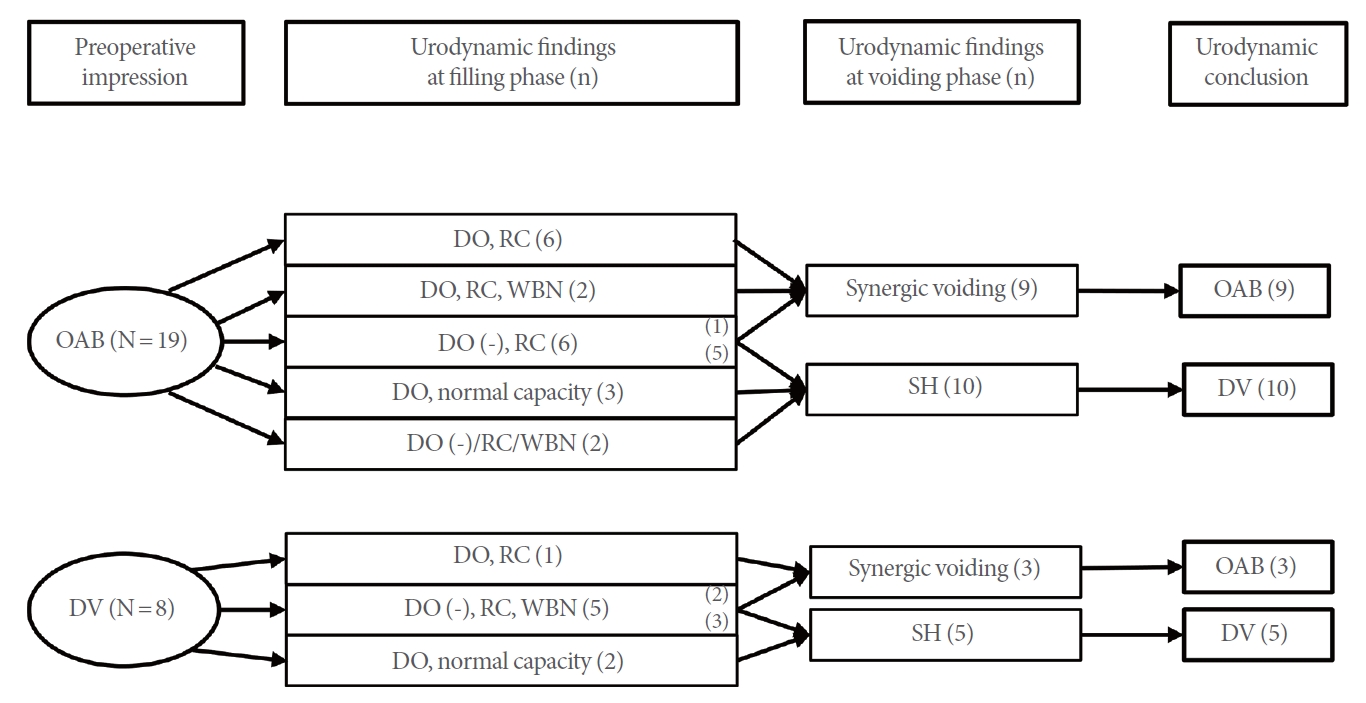
Videourodynamic study results for the included patients. OAB, overactive bladder; DO, detrusor overactivity; RC, reduced capacity; WBN, widening of the bladder neck; SH, sphincter hyperactivity; DV, dysfunctional voiding.
The consistency between clinical and urodynamic diagnoses was unsatisfactory, with an overall concordance rate of 52%. The sensitivity and specificity of the clinical versus urodynamic diagnoses were 75% and 33%, respectively.
Given our plan to treat patients according to their VUDS findings, intravesical and intrasphincteric BTX injections were attempted for urodynamic DO/RC and SH, respectively. Initially, only sphincteric BTX was attempted for the first 4 patients with SH, 2 of whom exhibited mild DO. Assuming that SH was the main pathophysiology, we believed that addressing only SH would sufficiently relieve enuresis. However, after recognizing unsatisfactory results among patients who received only a sphincteric BTX injection, the remaining 9 patients with DV who had SH also received an intravesical BTX injection. Fig. 2 summarizes the treatments provided to patients and enuresis outcomes at the 1-year follow-up.
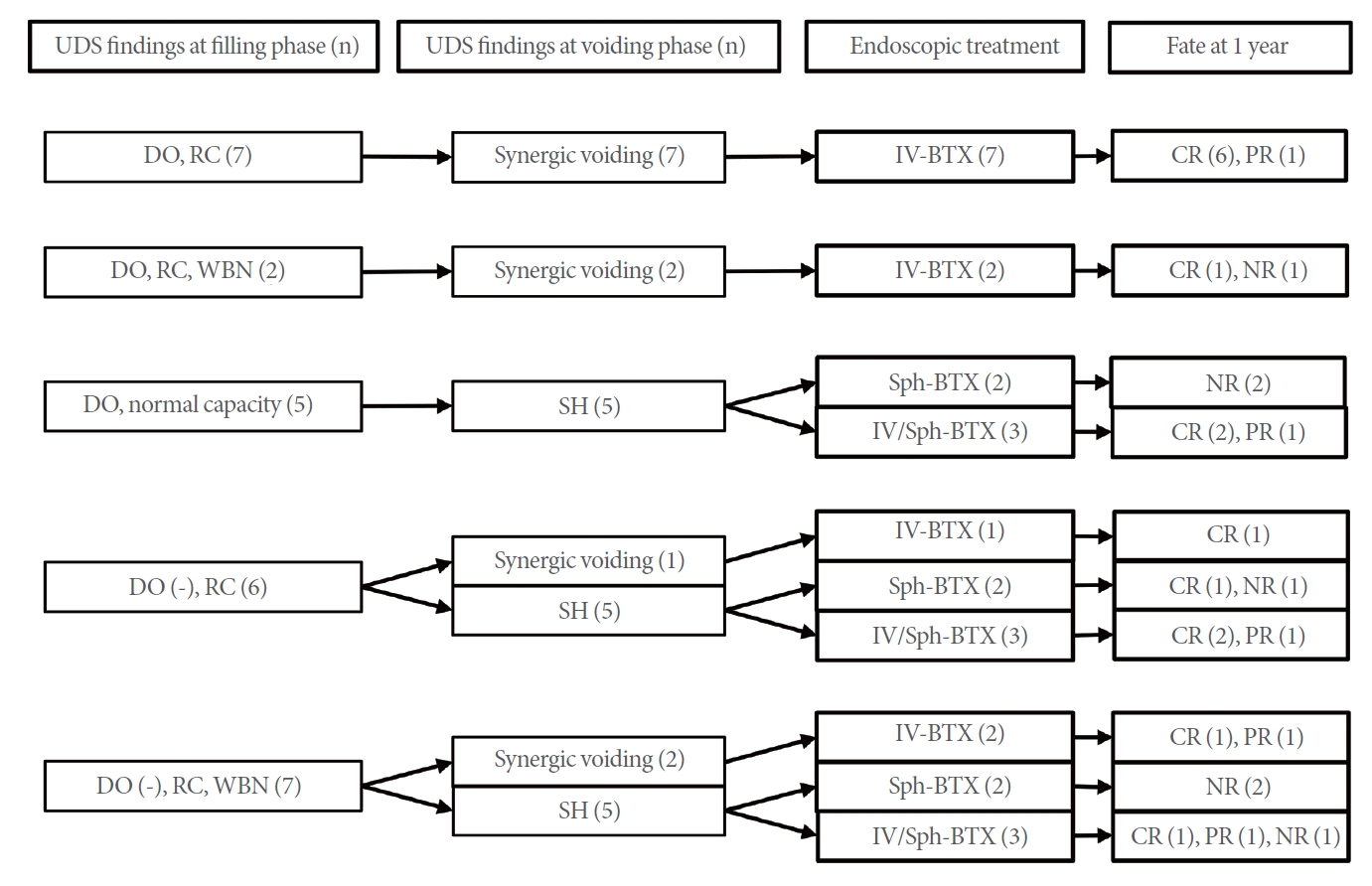
The treatments provided to patients and enuresis outcomes at the 1-year follow-up. UDS, urodynamic study; DO, detrusor overactivity; RC, reduced capacity; WBN, widening of bladder neck; SH, sphincter hyperactivity; IV, intravesical; Sph, sphincter; BTX, botulinum toxin; CR, complete remission; PR, partial remission; NR, no response.
Fig. 3 reveals that the time course for responses differed according to the type of urethral movement during voiding. Accordingly, patients showing synergic urethral movement showed a better treatment course than those showing SH. All but 1 synergic patient showed at least improvement of enuresis, while 75% experienced complete response (CR). Patients showed a transition from partial response (PR) or no response (NR) to CR after 6 months of treatment. In contrast, the initial responses worsened in the SH group, with 6 patients (40%) eventually showing NR. Although 11 patients (73%) showed CR or PR after 1 month of treatment, 2 patients with PR experienced a loss in efficacy, leading to NR. However, only 1 patient with PR transitioned to CR at 1 year. The responses stabilized 6 months after the injection.
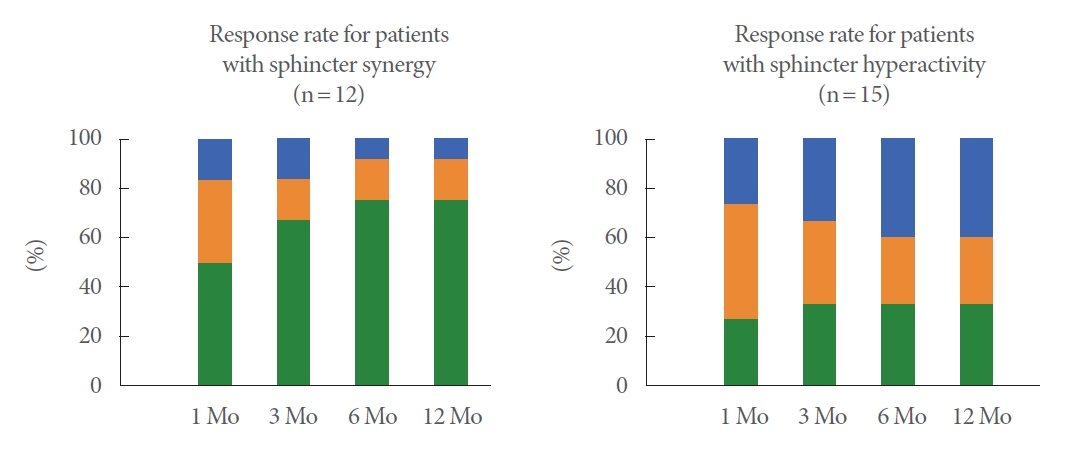
Time courses of responses according to the type of urethral movement during voiding. Blue color, no response; orange color, partial response; green color, complete response.
Apart from patients with CR who no longer required any treatment, 3 patients showing PR were able to control their enuresis with desmopressin or imipramine treatment.
Seven patients with NR (1 with synergic voiding and 6 with SH) failed to show any meaningful improvement. Most of them did not receive intravesical BTX injections. Moreover, 4 patients (2 of whom were girls) exhibited WBN in the filling phase and suffered persistent daytime incontinence and enuresis. One was found to have DO and synergic voiding, while the others showed SH.
Neither significant anesthesia-related nor acute treatment-related complications were observed. No postoperative complications were noted during follow-up.
DISCUSSION
The current study presented several urodynamic findings and combinations thereof that could explain treatment refractoriness. Our results showed that choosing the injection site based on urodynamic findings yielded a response rate of more than 70%. Furthermore, improvements were maintained for up to 12 months. As such, performing endoscopic treatment with BTX based on urodynamic findings within a single day can be considered an effective salvage treatment strategy.
All patients included herein had been diagnosed with NME, which is considered difficult to treat and requires prolonged treatment [18]. This is understandable since the treatment of NME requires the treatment of both LUTD and enuresis. An important caveat is that LUTD consists of heterogeneous urodynamic abnormalities. Thus, individual patterns of LUTD should be clarified and treated accordingly. However, the current management schemes are mainly nonspecific and noninvasive. As revealed by the European Bladder Dysfunction Study, the clinical diagnosis of OAB or DV was inconsistent with urodynamic results, while treatment aimed at either condition showed cross-efficacy with the other [19,20]. This redundancy in diagnosis and treatment supports the current management systems, which we acknowledge as helpful in most cases. However, establishing the correct diagnosis and providing tailored treatment may be needed, especially among refractory cases such as those included herein.
Our data confirmed that noninvasive test results could not predict the features observed during VUDS. Accordingly, only about half of the patients showed consistency between preoperative assumptions and the urodynamic diagnosis. We believe that the diagnostic discrepancy between clinical features and VUDS could partly explain instances of treatment failure. Moreover, 5 patients showed urodynamic features comprising both DO and SH, which shared features of OAB and DV. Such results cannot be determined from noninvasive diagnostic methods.
Unlike previous negative assertions about the utility of UDS for patients with refractory enuresis [9,21], all patients included herein showed at least 1 abnormal finding, highlighting the usefulness of VUDS. The most notable finding was that approximately half of those with clinically OAB turned out to have SH. This finding is consistent with previous reports of increased numbers of DV among refractory cases [22,23]. Although the application of fluoroscopy facilitated better identification of SH, the lack of DO during UDS did not seem to justify withholding treatment for DO, as shown by the higher prevalence of NR among those who received only intrasphincteric BTX for urodynamic DV than among those who received both intravesical and intrasphincteric BTX. Glassberg et al. [24] indicated that patients with DV should receive anticholinergics to alleviate storage symptoms despite the absence of DO during VUDS.
Gradual WBN during the filling phase was observed in 9 patients (35%) and was associated with daytime incontinence. Although WBN has not been identified as an independent predictor of incontinence, our results suggest that WBN may play a role in the development of treatment resistance. Of the 9 patients with gradual WBN during the filling phase, 4 finally resulted NR, of which 1 showed synergic voiding and 3 showed SH. Considering that the other 3 patients exhibiting WBN reported CR after treatment, WBN may not be the sole risk factor for treatment resistance. However, the finding that WBN was the only risk factor for nonresponsiveness in 4 patients may provide insights into its inhibitory role against continence. We have no knowledge of whether WBN is related to pelvic floor laxity [25] or an intrinsic sphincter problem [26]. However, a previous study reported that colposuspension successfully treated girls with congenital bladder neck insufficiency [26].
Unlike adults, most of the children who responded to treatment required just a single BTX shot. This is interesting since the effects of intravesical BTX are unlikely to last for more than 1 year. We speculate that the endoscopic injection was helpful for correcting DO and DV, which may be evidence of immaturity in bladder control, and facilitated the achievement of normal bladder or urethral control to such a degree that further correction by injection was not necessary.
As with other small case series, this study is subject to the problems of retrospective research. However, given the nature of enuresis, for which most conventional treatments show a good response, our data provide valuable guidance in managing refractory cases. Although we did not reveal an association between UDS findings and those of noninvasive studies, we did not check the relationship between invasive UDS and noninvasive uroflowmetry with concomitant EMG (EMG-UFM), which is useful for describing childhood voiding dysfunction. As the role of SH is highlighted in the development of refractory enuresis, the correlation of cystometric findings with EMG-UFM during uroflowmetry may obviate the need for invasive studies in the future.
In conclusion, the present study suggests that utilizing VUDS for patients with refractory enuresis with LUTD can help reveal the underlying pathophysiology and determine how to address the problem. VUDS was especially helpful in revealing sphincter pathology. Moreover, BTX injections were quite effective in controlling enuresis when administered in accordance with VUDS results. The beneficial effects of BTX were maintained once symptoms stabilized after 6 months of treatment.
Notes
Research Ethics
This study was approved by the Institutional Review Board (IRB) of Seoul National University Hospital Biomedical Research Institute (IRB No.1509-093-705).
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTION STATEMENT
·Conceptualization: GJ, KP
·Data curation: GJ, YI, GJ, JKS, KP
·Formal analysis: GJ, JKS
·Funding acquisition:
·Methodology: GJ, YI, JKS, KP
·Project administration:
·Visualization: GJ, GJ, JKS
·Writing-original draft: GJ
·Writing-review & editing: GJ, YI, KP
