|
 |


- Search
| Int Neurourol J > Volume 26(1); 2022 > Article |
|
ABSTRACT
Purpose
To develop a rat model of bladder calculi in the neurogenic bladder following spinal cord injury (SCI) and assess bacterial communities within the biofilm of bladder calculi using denaturing gradient gel electrophoresis (DGGE).
Methods
The silk tied to a small segment of the Teflon IV catheter was implanted through the urethra into the bladder of rats with SCI induced by T9 laminectomy. After 6 months, the rats were sacrificed and their bladder calculi were collected by opening the bladders through the low-midline incision. Genomic DNA was extracted from the biofilm of bladder calculi followed by DGGE to obtain bacterial DNA. The DNA sequences were compared and analyzed using BLAST (Basic Local Alignment Search Tool) to identify bacteria.
Results
After placing silk nidus in the bladder for 6 months, all 6 rats developed bladder calculi. According to DGGE analysis, Pseudomonas aeruginosa was the most dominant strain, while Clostridium sp. and Lactobacillus sp. were relatively dominant strains within the biofilm of bladder calculi in the rats with SCI.
- In the neurogenic bladder rat model, bacterial communities within the biofilm of the bladder calculi were analysed using the DGGE technique.
- This study will be the basic data for clinical research with the patients of spinal cord injury.
Bladder calculi are one of the most common urological problems in patients with neurogenic bladder (NB) following spinal cord injury (SCI), usually occurring within one year of injury [1,2]. A previous study found that 36% of SCI patients had bladder calculi during an 8-year follow-up period [1]. Urinary tract infections (UTIs) play an important role in the formation of bladder calculi in patients with NB following SCI, and the use of an indwelling catheter is more closely related to calculi originating as encrustations [2,3]. The components of bladder calculi in patients with NB following SCI are mainly related to infections and are different from those of calculi that develop in able-bodied elderly men with bladder outlet obstruction [4]. Since the development of bladder calculi in able-bodied individuals is relatively rare, there are only few studies on the pathogenesis and prevention of bladder calculi in patients with NB following SCI [5].
Bacteriological studies of urine and bladder calculi with traditional culture methods showed detection of Pseudomonas, Klebsiella, Staphylococcus, and Proteusi, with Escherichia coli being the most common; however, in about half of the cases, it was sterile [6]. With recent advances in molecular biology and culture techniques, microbial communities can be detected in urine, which was previously thought to be sterile in healthy individuals [7,8]. Although it has been difficult to detect bacteria that do not grow well in traditional cultures, these techniques have enabled the identification of fastidious bacteria that are difficult to cultivate [9,10].
Due to the difficulties caused by various host factors and external interferences in human studies, NB animal models are needed to understand the mechanisms of calculi pathogenesis and various changes that may affect bladder calculi formation [9,11]. Linsenmeyer and Ottenweller [5] performed SCI surgery 3 weeks after implantation of small pieces of catheters through an abdominal incision into the bladders of Sprague-Dawley rats, developing an NB rat model. All NB rats with implanted catheters had bladder calculi between 3 weeks and 3 months after SCI surgery. The components of bladder calculi in NB rats following SCI were similar to those in patients with NB following SCI, all of which were infection-related calculi.
We aimed to develop an NB rat model following SCI for the study of bladder calculi by modifying the previous rat model [5] by tying silk on a small segment of Teflon IV catheter as nidus and implanting them into the bladder through the urethra and assessing bacterial communities within the biofilm of bladder calculi using denaturing gradient gel electrophoresis (DGGE).
Female Sprague-Dawley rats (Koatech Inc., Pyeongtaek, Korea), weighing approximately 50–100 g and 3–4 weeks old, were used.
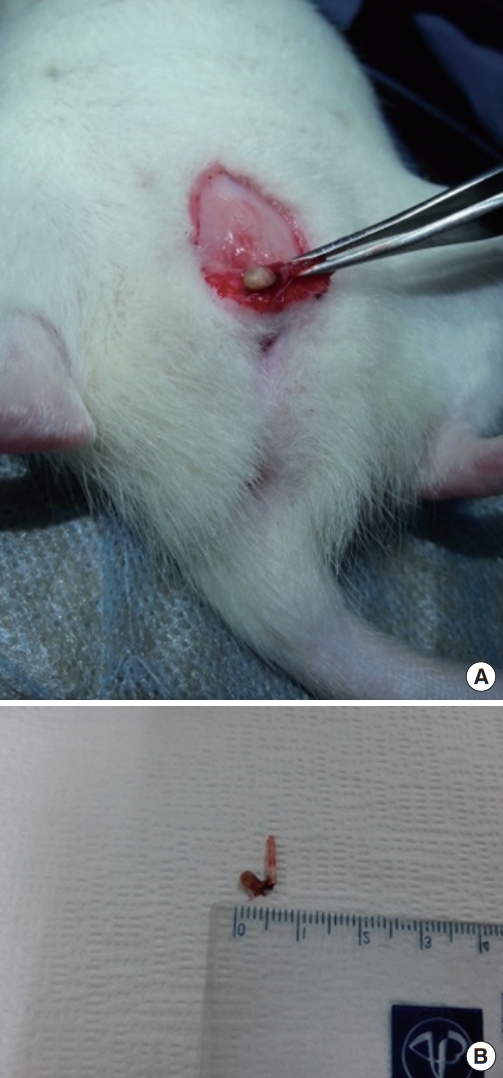
Rats were anesthetized by intraperitoneal administration of Zoletil (Tiletamin, Virbac Korea, Seoul, Korea) (0.2 mL). After exposing the T9 vertebrae, laminectomy was performed to make rats with NB following SCI. At the end of a small segment of a 24-G Teflon IV catheter, the silk was hung as nidus and implanted into the bladder through the urethra. After 6 months, the rats were sacrificed by intravenous administration of sodium pentobarbital (100 mg/kg) and their calculi and silk tied to a small segment of the Teflon IV catheter were collected by opening the bladder through the low-midline incision (Fig. 1).
The collected calculi were placed in plastic laboratory containers containing 10-mL phosphate-buffered saline (PBS) supplemented with 0.05% Tween 80 (polyoxyethylene sorbitan monooleate; Sigma-Aldrich, St. Louis, MO, USA). The calculi were manually fragmented with a sterile needle and shaken briefly. They were then sonicated for 90 seconds in a water-bath sonicator to dislodge the adherent microorganisms. Cells within the biofilm were harvested using sterile glass and washed once with PBS. The samples were centrifuged, and the supernatants were removed. Genomic DNA was then isolated from the harvested bacteria. DNA was transferred into 1.5-mL tubes containing 100 mL of 5% boiling Chelex 100 resin (Bio-Rad Laboratories, Hercules, CA, USA) and 20-mL Taq buffer, followed by incubation at 60°C for 30 minutes, heating in boiling water for 10 minutes, and cooling on ice for 3 minutes. The procedure was repeated 4–5 times, and the samples were centrifuged at 13,200 rpm for 10 minutes.
The extracted DNA was amplified using the bacterial universal primers 341F (5’-CCT ACG GGA GGC AGC AG-3’) and 907R (5’-CCG TCA ATT CCT TTG AGT TT-3’). The amplified DNA products were amplified again using 341F-GC (5’-CGC CCG CCG CGC GCG GCG GGC GGG GCG GGG GCA CGG GGG GCC-TAC GGG AGG CAG CAG-3’) to attach a 41-bp GC clamp and 907R for DGGE. Because the sequence length of the amplified DNA was 607 bp, 6% acrylamide was used, and 45% urea and 60% formamide were used. The concentration gradient for each experiment was chosen based on the optimal band pattern for each concentration. The final concentration of polymerase chain reaction (PCR) products was adjusted to over 200 ng/μL, of which, 40 μL was loaded into each lane. Gels were resolved at 65°C at 50 V for 16 hours. The patterns were compared with ladders using commercial software (InfoQuest FP SW; Bio-Rad Laboratories), and band intensities were analyzed using Quantity One 1-D analysis software (Bio-Rad Laboratories). DNA was extracted from the relevant bands, nested PCR was performed, and sequencing was outsourced to a service vendor (SolGent Inc., Daejeon, Korea). The sequences were compared and analyzed using BLAST (Basic Local Alignment Search Tool) (http://blast.ncbi.nlm.nih.gov/Blast.cgi) [9,12].
When silks tied to a small segment of the Teflon IV catheter were held in the bladder of rats with SCI for 6 months, all six rats had bladder calculi (Fig. 1).
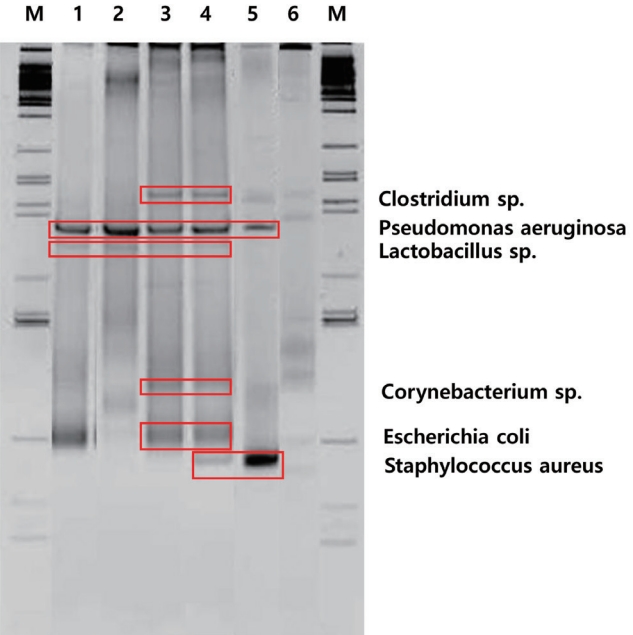
When DGGE performed on the biofilm of bladder calculi from each of the 6 rats with NB following SCI showed 6 bands. The sequencing of each band reveled bacterial strains Pseudomonas aeruginosa, Clostridium sp., Lactobacillus sp., Staphylococcus aureus, E. coli, and Corynebacterium sp. (Fig. 2). Based on the band density, P. aeruginosa was most evident, and Clostridium sp. and Lactobacillus sp. showed relatively clear patterns, indicating their dominance within the biofilm (Fig. 2).
Although the incidence of bladder calculi accounts for only 5% of all urinary calculi, it is the most common urinary complication in patients with NB following SCI and is primarily due to limited fluid intake, urinary stasis, bladder outlet obstruction, anatomical abnormalities, catheterization, or recurrent UTI [1,2,6]. Bladder calculi in patients with NB following SCI are mainly associated with UTI, and a variety of foreign bodies, such as Foley balloon fragments, hair, and silk sutures, can be involved in the formation of bladder calculi as nidus [13-15]. However, studies on how infections lead to the formation of calculi are lacking [5]. Human studies in patients with NB following SCI are challenged by host factors and external interference, including concomitant diseases, various methods of bladder emptying, and the use of prophylactic antibiotics [9,11]. An animal model for the study of bladder calculi in the NB following SCI was developed using Sprague-Dawley rats [5]. All six rats with NB following SCI with a 0.5-cm piece of latex 14F Foley catheter implanted into the bladder for 3 weeks to 3 months through an abdominal incision had bladder calculi [5]. The compositions of these calculi are similar to those of infection-related calculi in patients with NB following SCI [5]. The authors have reported bacterial communities with the biofilm of an IV catheter placed in the bladder through the urethra of female rats [9]. Therefore, we aimed to develop an animal model of bladder calculi that is easily reproducible through the urethra in female rats for the fundamental study of mechanisms and various changes that can affect the formation of bladder calculi. In our experiments, all 6 rats with NB following SCI with implanted silk tied to a small segment of the 24-G Teflon IV catheter into the bladder and maintained for 6 months had bladder calculi.
It is well known that UTIs are associated with the formation of bladder calculi in patients with NB following SCI [3]. Ureaseproducing bacteria, such as Proteusi, Klebsiella, Pseudomonas, and coagulase-negative staphylococci, play an important role in alkalizing urine and forming struvite calculi, but its pathophysiology has not been established [16,17]. In a study that conducted a bacteriological evaluation of urine and calculi from patients with bladder calculi [6], 24 out of 50 cases were negative in urine culture. The most commonly cultured microorganism was E. coli, but in some cases, different microorganisms were isolated from urine and calculi cultures. In studies that analyzed bacteria from the standard culture of the calculi themselves, bacteria were isolated in approximately 15%–70% of the calculi, and E. coli and Pseudomonas spp. were the most commonly isolated bacteria from calculi cultures [16]. A study that analyzed microbiological features of 203 febrile patients with upper urinary tract calculi revealed that the most commonly isolated bacteria from urine culture were E. coli (44.1%), followed by Enterococci spp. (11.8%), Proteusi spp. (8.6%), Streptococcus agalactiae (6.6%), Klebsiella spp. (5.3%), Pseudomonas spp. (4.6%), coagulase-negative staphylococci (4.0%), Staphylococcus epidermidis (4.0%), Serratia spp. (2.6%), Enterobacter spp. (0.7%), Acinetobacter spp. (0.7%), and mixed infections (7.2%) [18]. However, E. coli is not a urease-producing bacterium and is not thought to be involved in the formation of urinary calculi. Bacteriological results obtained from standard cultures of urine and calculi do not appear to reflect the bacteria involved in the formation of calculi in patients with bladder calculi [19].
Recent advances in culture techniques and molecular biology, such as enhanced quantitative urine culture and 16S rRNA gene sequencing, have revealed that there are a variety of microorganisms that are not well identified in standard urine cultures [7,8]. The combination of DGGE and PCR allows the identification of the DNA of various microorganisms present in the biofilm of urinary calculi, which can help determine how they play a role in the formation of bladder calculi [9,20]. As a result of the combination of DGGE and PCR, P. aeruginosa was the most dominant within the biofilm of bladder calculi from each of the six rats with NB following SCI. Clostridium sp. and Lactobacillus sp. were identified relatively dominant strains. This study is useful in that it developed an animal model for bladder calculi as the basis for clinical research, and showed that the DGGE technique and PCR are effective in identifying various microorganisms in the biofilm of calculi arising from NB.
The present study had several limitations. The Teflon IV catheter and silk used as nidus for the formation of bladder calculi in this animal model have limitations in reproducing the clinical situation in patients with NB following SCI. The composition of the bladder calculi obtained from this animal model was not analyzed. It has been reported that silk sutures can act as nidus in the formation of bladder calculi in patients with NB following SCI [15]. The composition of all calculi obtained from an animal model developed by implanting a piece of latex into the bladder of Sprague-Dawley rats with NB following SCI was infection-related calculi similar to those of patients with NB following SCI [5]. The animal model of bladder calculi developed in this study does not fully reflect all clinical issues associated with calculi in patients with NB, but is expected to provide basic data for clinical studies.
In conclusion, the Sprague-Dawley rat model implanted with silks tied to a small segment of the Teflon IV catheter after laminectomy seems to be an animal model suitable for the study of calculi in NB following SCI. Using DGGE analysis of the bacterial community, this study demonstrated various microorganisms in the biofilm of calculi arising from a NB rat model following SCI. This is useful in that it developed an animal model for bladder calculi as the basis for clinical research, and showed that the DGGE technique is effective in identifying various microorganisms in bladder calculi.
NOTES
REFERENCES
1. De Vivo MJ, Fine PR, Cutter GR, Maetz HM. The risk of bladder calculi in patients with spinal cord injuries. Arch Intern Med 1985;145:428-30. PMID: 3977510


2. Hansen RB, Biering-Sorensen F, Kristensen JK. Urinary calculi following traumatic spinal cord injury. Scand J Urol Nephrol 2007;41:115-9. PMID: 17454949


3. Burr RG. Urinary calculi composition in patients with spinal cord lesions. Arch Phys Med Rehabil 1978;59:84-8. PMID: 623518

4. Li WM, Chou YH, Li CC, Liuw CC, Huang SP, Wu WJ, et al. Local factors compared with systemic factors in the formation of bladder uric acid stones. Urol Int 2009;82:48-52. PMID: 19172097


5. Linsenmeyer TA, Ottenweller J. Bladder stones following SCI in the Sprague-Dawley rat. J Spinal Cord Med 2003;26:65-8. PMID: 12830972


6. Singh P. Bacteriological evaluation of bladder calculi: a study. Int J Res Med Sci 2018;6:1662-5. 
7. Kim M, Jung SI. The urinary tract microbiome in male genitourinary diseases: focusing on benign prostatic hyperplasia and lower urinary tract symptoms. Int Neurourol J 2021;25:3-11. PMID: 33504133



8. Thomas-White K, Brady M, Wolfe AJ, Mueller ER. The bladder is not sterile: history and current discoveries on the urinary microbiome. Curr Bladder Dysfunct Rep 2016;11:18-24. PMID: 27182288



9. Choe H, Kim HJ, Lee SJ, Lee JY, Lee SS, Cho YH. Evaluation of the bacterial distribution within the biofilm by denaturing gradient gel electrophoresis in the rat model of urinary catheters. Int Urol Nephrol 2013;45:743-8. PMID: 23563867


10. Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature 2006;444:1022-3. PMID: 17183309



11. Tzou DT, Taguchi K, Chi T, Stolller ML. Animal models of urinary stone disease. Int J Surg 2016;36:596-606. PMID: 27840313


12. Ishii K, Fukui M, Takii S. Microbial succession during a composting process as evaluated by denaturing gradient gel electrophoresis analysis. J Appl Microbiol 2000;89:768-77. PMID: 11119150


13. Matsuoka K, Nakagawa K, Eto K. A case history of a foreign body stone in the urinary bladder. Kurume Med J 1988;35:123-5. PMID: 3236824


14. Joshi M, Mittal N. Bladder calculi formed over a hair nidus in spinal injury cases. J Spinal Cord Med 2014;37:346-8. PMID: 24090316



15. Su CM, Lin HY, Li CC, Chou YH, Huang CH. Bladder stone in a woman after cesarean section: a care report. Kaohsiung J Med Sci 2003;19:42-4. PMID: 12693726

16. Schwaderer AL, Wolfe AJ. The association between bacteria and urinary stones. Ann Transl Med 2017;5:32. PMID: 28217697



17. Flannigan R, Choy WH, Chew B, Lange D. Renal struvite stones— pathogenesis, microbiology, and management strategies. Nat Rev Urol 2014;11:333-41. PMID: 24818849


18. Cho S, Park MG, Lee KC, Cho SY, Lee JW. Microbiological features and clinical factors associated with empirical antibiotic resistance in febrile patients with upper urinary tract calculi. J Korean Med Sci 2021;36:e3. PMID: 33398940


19. Lewi HJ, White A, Hutchinson AG, Scott R. The bacteriology of the urine and renal calculi. Urol Res 1984;12:107-9. PMID: 6377649


20. Arabski M, Stabrawa I, Kubala-Kukus A, Galczynska K, Banas D, Piskorz L, et al. The correlation of crystalline and elemental composition of urinary stones with a history of bacterial infections: TXRF, XRPD and PCR-DGGE studies. Eur Biophys J 2019;48:111-8. PMID: 30483831