Mediterranean Diet and Overactive Bladder
Article information
Abstract
Purpose
The relationship between nutrition and overactive bladder (OAB) has yet to be elucidated. Therefore, this study investigated the relationship between the Mediterranean diet and OAB.
Methods
The 14-item Mediterranean Diet Adherence Screener (MeDAS) and Overactive Bladder-Validated 8-question Screener (OAB-V8), validated in Turkish, were administered to 500 patients over the age of 18 who presented to outpatient clinics other than urology outpatient clinics. Of those patients, 174 with chronic diseases and urinary tract infections (based on urinalysis and a detailed medical history) were excluded. Therefore, 326 patients’ data were analyzed.
Results
There was a negative correlation between the MeDAS and OAB-V8 scores. High OAB-V8 scores were associated with obesity (body mass index ≥30 kg/m2), being single, and a low education level.
Conclusions
Dietary patterns represent a broader perspective on food and nutrient consumption and may therefore be more predictive of disease risk. The Mediterranean type should be recommended in the first-line treatment of patients with OAB symptoms. It is easily possible to determine the compliance of patients with this diet by using the 14-item MeDAS.
INTRODUCTION
Overactive bladder (OAB) syndrome is a symptom-based definition, in which urgency is the main complaint, usually accompanied by increased urination frequency and nocturnal urination, in the absence of any local pathological or metabolic reason to explain these symptoms [1,2]. The prevalence was reported as 20% in males and 35.7% in females in the most comprehensive population-based study conducted in Turkey. In the global literature, the reported prevalence is between 6.5% and 15.8% in men and between 9.3% and 32.6% in women. OAB symptoms increase with age in both sexes, and the associated costs will continue to increase as the average age of the population increases [3]. The estimated total economic cost of OAB in the United States was $12.02 billion in 2000, and OAB reduces the quality of life for millions of women [4].
OAB has been associated with obesity, smoking, fluid intake, the consumption of carbonated drinks, and diet. The first-line treatment is lifestyle and nutritional changes, and the priority is to improve patients’ quality of life [5-7]. Voiding is controlled by the sympathetic and parasympathetic nervous systems. Since inflammation and endothelial damage may play a role in urological symptoms, it is reasonable that changes in dietary patterns affect OAB [8,9]. Multiple hypotheses have been proposed regarding the pathophysiology of OAB. According to the detrusor overactivity hypothesis, peripheral afferent nerves become sensitized during bladder filling and urinary urgency request occurs without a change in detrusor pressure [10]. Poor diet and lifestyle are recognized causes of type 2 diabetes and obesity, with related metabolic dysfunction. In urodynamic studies, 55% of patients with diabetes had OAB due to vascular and peripheral nerve damage [11,12].
In recent years, many studies have examined the relationship between health and overall diet, and the effects of broad dietary patterns have been examined rather than focusing on individual foods. Dietary patterns represent a broader perspective on food and nutrient consumption and may therefore be more predictive of disease risk. The term “Mediterranean diet,” which refers to a more plant-based pattern of consumption, has been associated with improved survival. The main features of the Mediterranean diet include abundant vegetable intake, olive oil as a source of fat, the consumption of grains, legumes, and nuts with high amounts of pulp, low-medium consumption of fish and poultry, relatively low consumption of red meat, and normally moderate amounts of wine with meals [13]. Epidemiological studies have revealed the protective effect of the Mediterranean diet against mild chronic inflammation and metabolic complications. The Mediterranean diet model is rich in foods with positive anti-inflammatory properties and poor in proinflammatory foods [14,15]. We therefore hypothesized that the Mediterranean diet may be associated with OAB.
MATERIALS AND METHODS
We included 500 patients who presented to outpatient clinics other than the urology outpatient clinic in our study. From that sample, we excluded 174 patients with heart failure, chronic renal failure, diabetes, chronic obstructive pulmonary disease, neurological diseases, general cognitive disorders, sleep disorders (e.g., sleep apnea), depression, and urinary tract infection (based on urinalysis and a detailed medical history). The Turkish-validated Overactive Bladder-Validated 8-question Screener (OAB-V8) and the Turkish-validated 14-item Mediterranean Diet Adherence Screener (MeDAS) were used [16,17]. In the 14-item MeDAS, a score of 0 or 1 point is assigned for each question asked, and the total score is calculated. A total score of 7 and above indicates that the individual has an acceptable level of compliance with the Mediterranean diet, and a score of 9 and above indicates that the individual has strict compliance with the Mediterranean diet [16]. OAB-V8 Bladder Inquiry Form scores are aggregated to provide a global score ranging from 0 to 40. Scores from 0 to 8 are considered low, 8 to 16 as medium, and greater than 16 as high, which healthcare providers can take into consideration when making a diagnosis of OAB [17,18].
Statistical Analysis
The numerical data of our study were not normally distributed according to the Kolmogorov-Smirnov test. The Kruskal-Wallis test and Mann-Whitney U-test were used to compare demographic data and questionnaire data. Spearman correlation analysis was used to evaluate relationships between various variables. The statistical analyses were carried out using IBM SPSS Statistics ver. 27.0 (IBM Co., Armonk, NY, USA). A P-value of <0.05 was considered to indicate statistical significance.
RESULTS
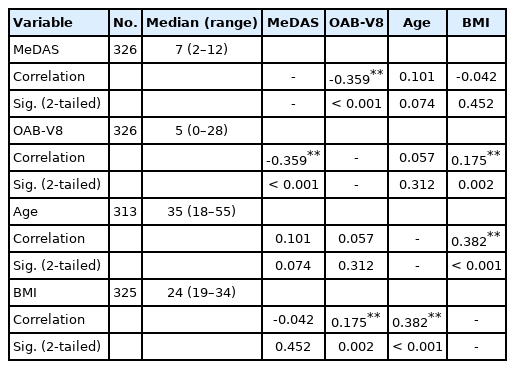
Initially, 500 people were recruited for our study, but 326 were included after applying the exclusion criteria. Demographic data are shown in Table 1. Sex (P=0.008) and marital status (P=0.047) showed statistically significant relationships with the MeDAS score; specifically, women and married people were more compliant with the Mediterranean diet. Smoking (P = 0.127), education level (P=0.074), and obesity (P=0.758) did not have statistically significant relationships with the MeDAS score. For the OAB-V8 score, statistically significant relationships were found for education level (P=0.001) marital status (P=0.023) and obesity (body mass index [BMI] ≥30 kg/m2) (P=0.049), but not for sex (P=0.478) or smoking (P=0.464). A high OAB-V8 score was associated with obesity (BMI ≥30 kg/m2), being single, and a low education level (Table 2).
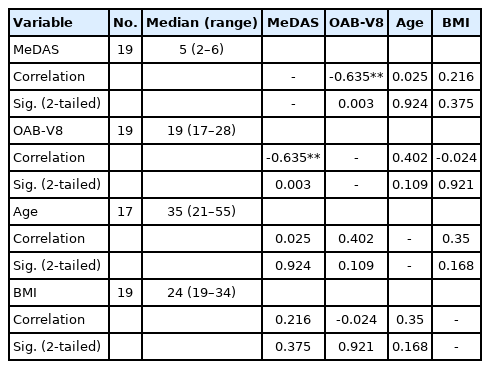
MeDAS scores (Fig. 1) and OAB-V8 scores (Fig. 2) were statistically negatively correlated (P<0.001) (r=0.359). OAB-V8 scores and BMI showed statistically positive correlations (P=0.002) (r =0.175). Age and BMI were significantly positively correlated (P<0.001) (r=0.382). Descriptive numerical data of age, BMI, MeDAS scores, and OAB-8 scores are shown in Table 3. When patients with OAB-V8 scores>16 (high scores according to the questionnaire) were evaluated as a separate group, the data were statistically significant. The correlation between the MeDAS score and the OAB-V8 score in this group was much stronger than the overall comparison (Table 4).

A histogram showing the distribution of Mediterranean Diet Adherence Screener (MeDAS) scores. SD, standard deviation.
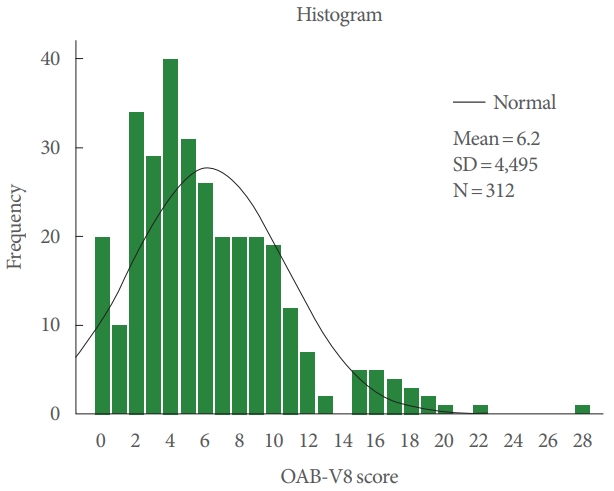
A histogram showing the distribution of Overactive Bladder-Validated 8-question Screener (OAB-V8) scores.
DISCUSSION
OAB is primarily a diagnosis of exclusion and a symptom complex. Its treatment is aimed at alleviating symptoms and cannot reverse pathophysiological abnormalities. Understanding the pathophysiology and risk factors for developing OAB is essential for both treating and preventing the syndrome. Studies evaluating the development, natural history, and progression of OAB are needed. The timing and circumstances in which OAB develops and its associated risk factors are not yet well understood [19,20].
No study has yet examined the relationship between OAB and a Mediterranean diet. However, there is increasing evidence that diet may play a significant role in the development of OAB symptoms. Dallosso et al. [21] collected data from 7,046 women over the age of 40 to investigate the role of diet and lifestyle in OAB and urinary incontinence. Information about the onset of OAB symptoms, diet, and lifestyle was obtained by a postal questionnaire. Significant relationships were observed between OAB symptoms and obesity, smoking, and carbonated drink consumption. The risk of OAB symptoms was reduced with higher consumption of vegetables, bread, and chicken [21]. In our study, adults of both sexes participated. Of the 500 initially recruited patients, 174 were excluded from the study based on a detailed medical history in outpatient clinics and the results of urinalysis. Chronic diseases and urinary tract infections, which constituted the exclusion criteria, could have resulted in misleading results regarding the diagnosis of OAB syndrome. Obesity has similarly been associated with OAB, as in the results reported by Dallosso et al. [21] based on an evaluation of food consumption in various patient groups. In our study, the cumulative effect of foods was highlighted by evaluating the MeDAS. Furthermore, Sexton et al. [22] found that education level and OAB symptoms were statistically associated. Similar results were obtained from the data in our study.
Another large population-based study including 2,060 women was reported based on the Boston Area Community Health Survey (2002–2005). Although there was no relationship of carbohydrate, protein, or fat intake with OAB, the ratio of saturated fat to polyunsaturated fat was positively correlated with incontinence. Another analysis in this cohort of patients showed that increased saturated fat intake was associated with postvoid symptoms, while high protein intake was associated with OAB symptoms. However, there was no consistent relationship with carbohydrate and monounsaturated/polyunsaturated fat intake [8]. Obesity has been associated with OAB, similar to the findings of our study. The Mediterranean diet is poor in saturated fat. For this reason, we interpret extant data as compatible with our study. However, dietary patterns represent a broader perspective on food and nutrient consumption and may therefore be more predictive of disease risk. Considering the setting of outpatient clinic visits, we think that the Mediterranean diet should be recommended as the first step in the treatment of OAB symptoms. A short information form will raise awareness of patients about the Mediterranean diet.
In conclusion, the Mediterranean diet should be recommended for the first-line treatment of patients with OAB symptoms. It is easily possible to determine the compliance of patients with this diet by using the 14-item MeDAS.
Notes
Research Ethics
This study was approved by the Ethics Committee of Manisa Celal Bayar University Faculty of Medicine Health Sciences (No. 10/02/2021/20.478.486/750).
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTION STATEMENT
·Conceptualization: YEB, GT, OÜ
·Data curation: YEB, GT, TM, OÜ
·Formal analysis: YEB, GT
·Methodology: YEB, GT, OÜ
·Project administration: YEB, TM
·Visualization: YEB
·Writing-original draft: YEB
·Writing-review & editing: YEB