Microtubule-Targeting Agents Enter the Central Nervous System (CNS): Double-edged Swords for Treating CNS Injury and Disease
Article information
Abstract
Microtubules have been among the most successful targets in anticancer therapy and a large number of microtubule-targeting agents (MTAs) are in various stages of clinical development for the treatment of several malignancies. Given that injury and diseases in the central nervous system (CNS) are accompanied by acute or chronic disruption of the structural integrity of neurons and that microtubules provide structural support for the nervous system at cellular and intracellular levels, microtubules are emerging as potential therapeutic targets for treating CNS disorders. It has been postulated that exogenous application of MTAs might prevent the breakdown or degradation of microtubules after injury or during neurodegeneration, which will thereby aid in preserving the structural integrity and function of the nervous system. Here we review recent evidence that supports this notion and also discuss potential risks of targeting microtubules as a therapy for treating nerve injury and neurodegenerative diseases.
INTRODUCTION
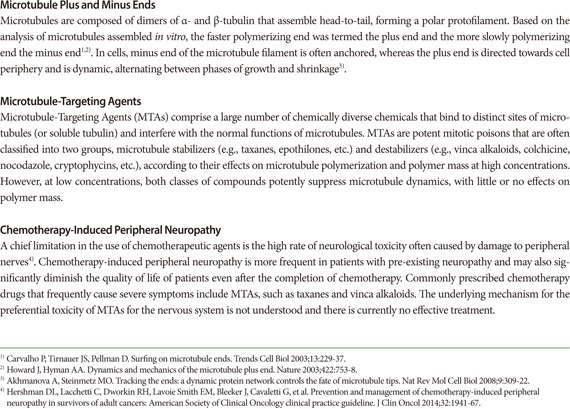
Microtubules are major components of the cytoskeleton in all cells. They are dynamic macromolecular structures composed of polarized tubulin dimers with fast-growing plus ends and more stable minus ends, usually organized in a radial array with plus ends directed toward the cell periphery (see Glossary Appendix). Microtubules form a mechanical framework throughout the cell and play key roles in a variety of cellular processes, such as cell proliferation, migration, and differentiation. For this reason an expanding list of microtubule-targeting agents (MTAs) has been developed for different purposes, including for use as antifungal agents, pesticides, and anticancer agents (see Glossary Appendix).
It is well known that in dividing cells, microtubules constituting the mitotic spindle are highly dynamic and are extremely susceptible to perturbations. Such hypersensitivity of dynamic microtubules to perturbations during cell division provides an explanation for why targeting microtubules with MTAs have proven to be highly effective in patients with cancer [1]. In addition to dividing cells, postmitotic neurons harbor microtubules that undergo dynamic assembly and disassembly, clustering and splaying, and local stabilization and destabilization. In recent years, translational neuroscientists have developed an intriguing hypothesis proposing microtubules as a promising therapeutic target for treating nervous system injury or neurodegenerative diseases [2,3,4]. Given that injury and disease of the nervous system are often accompanied by acute or chronic disruption of the structural integrity of neurons and that microtubules provide structural support for the nervous system at cellular and intracellular levels, it has been suggested that exogenous application of MTAs might prevent the breakdown or degradation of microtubules after injury or during neurodegeneration, which will thereby aid in preserving the structural integrity and function of the nervous system. Here we review recent proof-of-principle experiments that support this notion and also discuss potential risks of targeting microtubules as a therapy for treating nerve injury and neurodegenerative diseases.
NEURONAL MICROTUBULES
The dynamic lengthening and shortening of spindle microtubules that mediate chromosome segregation during cell division is an iconic process illustrating the dynamicity of microtubules. Microtubules in nonneuronal cells are in constant dynamic flux being taken apart and rebuilt, consistent with their need to rapidly reorganize during cell division, migration, and differentiation. Such dynamic microtubules are susceptible for perturbations, such as exposure to cold and depolymerizing drugs. By contrast, neuronal microtubules are resistant to depolymerizing conditions, which would normally cause microtubules to disassemble in nonneuronal cells [5], and the presence of such 'stable' microtubules have long been thought to provide a structural framework for the complex neuronal architecture. Moreover, given the highly compartmentalized nature of neurons, neuronal function heavily relies on active transport and communication between the cell body and the axon, and thus structural integrity is essential for neuronal microtubules to serve as stable tracks for transport of proteins, vesicles and organelles over long distances [6].
Contrary to conventional wisdom that neuronal microtubule network is composed of stable microtubules, the discovery of a specific class of microtubule-associated proteins, known as plus end-interacting proteins (+TIPs) has revealed that a fraction of neuronal microtubules is quite dynamic [7,8]. Recently, in vivo imaging of +TIPs that bind specifically to actively growing microtubule plus ends confirmed that neuronal microtubules exhibit dynamicity even in fully mature neurons, as in nonneuronal cells [8]. Increasing lines of evidence suggest that such dynamic nature of neuronal microtubules is essential for normal function: it is crucial not only during development to organize and construct the complex nervous system, but also in adulthood to maintain the ability to undergo structural rearrangement required for synaptic plasticity. It appears that tight spatial and temporal regulation of microtubule assembly/disassembly, bundling/splaying, stability/dynamics is key to function both during development and adult life, and multiple layers of regulations exist to achieve the balance between opposing properties of microtubules. To reconcile rather conflicting needs for dynamics and stability, it has been suggested that neurons contain multiple classes of microtubule polymers that differ in stability [9,10] and that regions of stable, less stable, and labile microtubules might exist as distinct domains on individual microtubules [11]. In that way, most stable microtubule fraction unique to neurons would serve to preserve the organization, whereas the dynamic class residing in specific regions might be responsible for the structural modification in response to physiological stimuli.
Given the complex organization and intricate regulation of microtubules in neurons, it is no surprise that dysfunction of the microtubule network has been associated with a number of nervous system disorders. Disruption of the structural integrity of the microtubule network and interruption of microtubule function are observed after traumatic injury in the nervous system [8,12], as well as in a number of neurological disorders, ranging from neurodevelopmental disorders to neurodegenerative diseases [13,14,15,16]. Accordingly, stabilization of microtubules has emerged as a possible therapeutic approach for treating injuries and diseases in the central nervous system (CNS). This expectation has gathered more attention with the development of MTAs that penetrate the blood-brain-barrier (BBB), such as epothilones, and noscapine and analogues. The feasibility of such approach is currently under evaluation by applying clinically approved MTAs in animal models of CNS injury or neurodegenerative diseases, as discussed in the following sections.
MICROTUBULES ARE POTENTIAL TARGETS FOR ENHANCING AXON REGENERATION AFTER TRAUMATIC INJURY
Traumatic injuries to the CNS can sever axonal tracts and lead to functional deficits. The degree of functional loss varies, but CNS injuries have the potential to permanently debilitate the victims, largely because damaged axons in the CNS fail to regrow after injury. Inability to restore the compromised connections is due to both the diminished intrinsic growth state of a neuron and the hostile extracellular environment of the CNS [17,18]. Intrinsically, neurons undergo a developmental decline in the potential to grow axons, and extrinsically, severed axons encounter a milieu of growth impediments, exemplified by the presence of inhibitory molecules comprising the glial scar [17,19]. Of note, microtubule dynamics play a part in the regulation of both the intrinsic growth state and the formation of a glial scar. On the one hand, severed axons form dystrophic retraction bulbs at the ends, which are morphological hallmarks of lesioned axons that fail to regrow [12]. Disorganized microtubules have been suggested to underlie the formation of retraction bulbs and subsequent regeneration failure [12]. Indeed, reorganization of microtubules in the severed axon was sufficient to prevent the generation of retraction bulbs, and subsequently promoted axon growth over potent growth impediments [20]. On the other hand, microtubule dynamics regulate key processes of scarring, including cell proliferation, migration, as well as intracellular trafficking and secretion of extracellular matrix molecules [3]. Thus, adequate control over microtubule dynamics is likely to yield beneficial outcomes by enhancing intrinsic axon growth ability and alleviating glial inhibition.
Recent studies have presented proof-of-principle experiments supporting the notion that modulation of microtubule dynamics can enhance axon regeneration. Hellal et al. [3] delivered paclitaxel directly to the injury site in a rodent model of spinal cord injury and found that local administration of low-dose paclitaxel promoted axon regeneration. Paclitaxel treatment could reduce scar formation and induce the growth of serotonergic (5HT) axons. Importantly, these anatomical changes were accompanied by recovery of motor function [3], suggesting an exciting possibility of exploiting the growing collection of MTAs to the treatment of CNS repair. Similarly, application of paclitaxel directly around the lesion site improved axon regeneration after optic nerve crush when combined with a lens injury [21], a procedure that transforms retinal ganglion cells into a growth-competent state [22]. Local administration, but not intravitreal injection, of paclitaxel delayed scar formation, prevented macrophage infiltration into the lesion site, and enhanced axon regeneration [21], further supporting the notion that manipulation of microtubules at the injury site can yield beneficial outcomes. However, in a recent study, intrathecal infusions of paclitaxel did not promote axon regeneration nor did it improve functional recovery in a rodent model of spinal contusion injury, although administration of paclitaxel did reduce scarring [23]. These disparate results imply that a comprehensive and mechanistic understanding is needed before moving forward with translation of MTAs for repair after CNS injuries. Furthermore, it should be noted that, in the aforementioned studies [3,21] paclitaxel had to be delivered directly to the lesion site to produce desired outcome, because this drug cannot cross the BBB. Application of paclitaxel for treating CNS injury, thus, has limited therapeutic value, as local administration of a drug directly to the injury site often requires invasive procedures, which can induce further CNS damage. Therefore, it will be intriguing to examine if axon regeneration and neural repair can be promoted by administration of MTAs that penetrate the BBB.
TARGETING MICROTUBULES AS AN APPROACH FOR TREATING NEURODEGENERATIVE DISEASES
Neurodegenerative diseases show common pathological features, including abnormal protein aggregation, mitochondrial dysfunction, and disease-specific neuronal degeneration. Interestingly, several pathogenic proteins, such as Tau, α-synuclein, Parkin, leucine-rich repeat kinase 2 (LRRK2), and Huntingtin, related to neurodegenerative diseases have been reported to directly bind tubulin or modulate microtubule stability. Currently, increasing lines of evidence suggest that MTAs can ameliorate the pathogenic symptoms in animal models of neurodegenerative diseases. In addition to administrating drugs that directly stabilize microtubules, strategies to tackle microtubule-based transport system are also under development, as impairment in the axonal transport has recently emerged as a common factor in several neurodegenerative diseases [6,24]. It remains to be determined whether interventions that correct transport deficits can confer therapeutic effects and slow the progression of neurodegenerative diseases.
Alzheimer Disease or Tauopathies
The presence of intraneuronal neurofibrillary tangles (NFT) composed of Tau is a pathological marker found in multiple neurodegenerative diseases, including Alzheimer disease (AD), frontotemporal dementias, corticobasal degeneration, progressive supranuclear palsy, Pick disease, and parkinsonism-linked chromosome 17 [25]. Under normal physiological conditions, Tau is enriched in axons and functions to stabilize microtubules. However, under pathological conditions, such as AD, Tau progressively detaches from microtubules and becomes hyperphosphorylated [26,27]. It has been postulated that the abnormal dissociation of Tau from microtubules disrupts the dynamics and organization of axonal microtubules, which can lead to microtubule destabilization, axonal transport deficits, and neurodegeneration. Accordingly, attempts have been made to alleviate pathological defects of neurodegenerative tauopathies by administrating microtubule-stabilizing MTAs. To test this possibility, paclitaxel was applied to Tau transgenic mice that develop NFT-like inclusions in the brain stem and the spinal cord [4]. Drug absorption in Tau transgenic mice at neuromuscular junctions increased microtubule density and enhanced axonal transport in spinal motor neurons, which project to striated muscles where there is no BBB [4]. Unfortunately, applying paclitaxel is limited in other animal models of tauopathies, which more closely recapitulate human pathology, due to the poor BBB penetration of paclitaxel.
Applying MTAs to treat CNS diseases has become more feasible by the development of MTAs that cross the BBB, such as epothilone D [28]. In PS19 transgenic mice-which develop forebrain accumulations of insoluble Tau inclusions, axonal degeneration, and neuronal loss-intraperitoneal injections of epothilone D significantly reduced dystrophic axons and increased microtubule density [29]. Barnes maze test of learning and memory revealed that such changes in axonal architecture were accompanied by improvement of cognitive performance in the PS19 mice [29]. These proof-of-principle experiments suggest brain-penetrant MTAs as potential treatments for AD and other neurodegenerative tauopathies.
Parkinson Disease
In general, Parkinson disease (PD) patients show defective locomotive movement caused by the reduction of dopamine contents in the striatum. Degeneration of nigrostriatal dopaminergic synaptic terminals precedes the loss of dopaminergic neurons in the substantia nigra [30]. A variety of environmental and genetic factors have been documented to cause such selective loss. Exposure to environmental factors, such as rotenone and 1-methyl-4-phenylpiridinium (MPP+), either by systemic or chronic administration induces PD-like symptoms and degeneration of dopaminergic neurons in animal models. The neuronal loss induced by these factors has been suggested to occur through inhibition of mitochondrial complex I and depolarization of microtubules [31]. The disruption of microtubule network has been shown to induce the accumulation of dopamine vesicles at the soma, which causes oxidative damage by the oxidation of leaked dopamine from the accumulated dopamine vesicles [31]. Supporting the relevance of disrupted microtubule network in rotenone-induced parkinsonism, the selective loss of dopaminergic neurons induced by rotenone could be mimicked by other microtubule-depolymerizing agents, such as colchicine or nocodazole, and the loss of dopaminergic neurons could be reversed by applying paclitaxel which stabilizes microtubules [31]. Furthermore, epothilone D has been shown to prevent defects in microtubules and attenuate the degeneration of nigrostraial track induced by MPTP, a precursor of MPP+ [32].
In addition to environmental toxins, several proteins related to PD, such as α-synuclein, Parkin, and LRRK2, have been shown to directly bind tubulin (or microtubules) and modulate microtubule stability [33,34,35]. Especially, overexpression of α-synuclein and pathogenic mutants of LRRK2 resulted in increasing Tau phosphorylation, and axonal transport deficits induced by LRRK2 could be reversed by applying a tubulin deacetylase inhibitor, trichostatin A [36]. Parkin, a protein E3 ligase linked to PD, is known to ubiquitinate α- and β-tubulin, leading to their degradation through activation of the proteasomal pathway [37]. The physiological significance of the interaction between microtubules and several proteins encoded by genes associated with PD are currently not fully understood, but such interactions might play a part in regulating microtubule stability and microtubule-based transport in neurons.
CHALLENGES
Lessons Learned From Cancer
Microtubules have been among the most successful targets in anticancer therapy and a large number of MTAs are in various stages of clinical development for the treatment of several malignancies [1]. As such, this class of drugs has been intensively scrutinized, revealing compound-specific or rather common side-effects. The clinical success of MTAs has often been hampered by the risk of inducing peripheral neuropathy, commonly referred to as chemotherapy-induced peripheral neuropathy [38] (see Glossary Appendix). Given systematically at cancer-reducing dosages, treatment with pactitaxel and related drugs frequently produces painful neuropathies and can affect quality of life even after the completion of chemotherapy [38,39,40]. Peripheral sensory nerves are especially sensitive to MTA exposure, which might be associated with their long length of axons, but the hypersensitivity of sensory neurons to MTAs is not understood at a mechanistic level. There have been few reports of CNS toxicity so far with the currently prescribed MTAs, presumably because of the fact that a large number of MTAs have poor BBB permeability. However, it is possible that the more recently approved MTAs, especially those that penetrate the BBB, might be associated with, or cause CNS toxicity. Therefore, studies aiming at testing the feasibility of applying BBB-penetrating MTAs for the treatment of neurodegenerative diseases should also thoroughly examine whether such administration affects the intact nervous system, both the CNS and the PNS. In this regard, the development of reliable and predictive models of neurotoxicity should be of great use.
Fundamental Challenges of Microtubule-based Approaches
Aside from drug toxicity, the multiple roles of microtubules in the plethora of cellular activities raise a fundamental issue of microtubule-based approaches related to specificity. Given that microtubules are essential components in all cells, it is possible that MTAs delivered to the system with the purpose of acting on neuronal microtubules also substantially affect microtubules in other cells. To gain insights into this issue, it will be of paramount importance to understand why systematic application of anticancer agents preferentially affects sensory neurons in the PNS, how neuronal microtubules differ from those of non-neuronal cells, and how neuronal microtubules and microtubule-based transport system are regulated.
However, it is also possible that, by interfering multiple processes, microtubule-based approaches might induce the desired effect to the entire biological system, especially in the context of CNS injury and diseases where injury responses or development of diseases are quite complex. As discussed above, paclitaxel could promote neural repair by promoting axonal regrowth, decreasing scar formation, and reducing macrophage infiltration, effects that result from modulation of multiple pathways through regulation of microtubules not only in neurons, but also in nonneuronal players in the nervous system [3,21]. Therefore, targeting microtubule dynamics might offer an effective way to control multiple pathways or processes, and thereby produce better outcomes than single-target approaches aiming at controlling one particular cellular process.
CONCLUDING REMARKS AND FUTURE PERSPECTIVES
MTAs have been widely prescribed as components of chemotherapy. In addition to treating malignancy, translational neuroscientists are pointing to MTAs as promising therapeutic agents for treating injury and disease in the CNS. In this respect, MTAs that cross the BBB are gathering particular attention. However, a comprehensive evaluation of the different classes of MTAs in the context of neurological disorders and diseases has not yet been achieved. Given the well-known peripheral neuropathy induced by chemotherapy, a critical challenge facing the development of CNS-directed microtubule-stabilizing therapies is finding ways to specifically retain MTAs in the CNS to allow for prolonged drug activity where needed, while minimizing sustained peripheral exposure.
It is obvious that advances in understanding the function and regulatory mechanism of neuronal microtubules has high clinical and public health significance and will lead to improved targeting and development of therapeutic interventions. The risks and benefits of targeting microtubules should be thoroughly considered to aid in planning future investigations. We hope that as we gain more knowledge and insight into the molecular mechanisms of action of MTAs and regulatory mechanisms of neuronal microtubules, we will be able to design better ways to target microtubules and produce desired clinical activities.
ACKNOWLEDGEMENTS
We apologize to authors whose work could not be cited or cited only through review articles due to space constraints.
Notes
This study of E.M.H. is supported by the Institutional Grant of the Korea Institute of Science and Technology (2V03430). This study of B.D.L is supported by the grant of NRF (NRF-2012R1A1A1010564, NRF-2012-0001161) funded by Korea Ministry of Science.
No potential conflict of interest relevant to this article was reported.
Appendices
Appendix 1
GLOSSARY