Influence of Panax ginseng on Alpha-Adrenergic Receptor of Benign Prostatic Hyperplasia
Article information
Abstract
Purpose
Benign prostatic hyperplasia (BPH) is the most common prostate problem in older men. The present study aimed to investigate the inhibitory effect of Panax ginseng C.A. Meyer ( P. ginseng) on a rat model of testosterone-induced BPH.
Methods
The rats were divided into 3 groups (each group, n=10): control, testosterone-induced BPH (20 mg/kg, subcutaneous injection), and P. ginseng (200 mg/kg, orally) groups. After 4 weeks, all animals were sacrificed to examine the blood biochemical profiles, prostate volume, weight, histopathological changes, alpha-1D adrenergic receptor (Adra1d) mRNA expression, and epidermal growth factor receptor (EGFR) and B-cell CLL/lymphoma 2 (BCL2) protein expression.
Results
The group treated with P. ginseng showed significantly lesser prostate size and weight than the testosterone-induced BPH group. In addition, P. ginseng decreased the mRNA expression of Adra1d as well as the expression of EGFR and BCL2 in prostate tissue.
Conclusions
These results suggest that P. ginseng may inhibit the alpha-1-adrenergic receptor to suppress the development of BPH.
INTRODUCTION
Benign prostatic hyperplasia (BPH) is a common urological disease in aged men. It is characterized by benign enlargement of the prostate. The male urethra runs through the prostate, and therefore an enlarged prostate may constrict the urethra and cause lower urinary tract symptoms (LUTS), including difficult and weak urination, frequent voiding, nocturia, dysuria, and bladder obstruction [ 1]. These symptoms can negatively affect the quality of life of BPH patients. BPH generally does not occur before the age of 30, but it often develops between the ages of 30 and 80. By 80 years of age, approximately 90% of men would develop BPH [ 2]. Late treatment of prostatic hyperplasia leads to several issues, such as acute urinary retention, renal insufficiency, urinary tract infection, gross hematuria, bladder stones, and kidney failure [ 3, 4, 5].
Although the pathogenesis of BPH is not exact, previous studies have reported that sex hormones, including androgen, testosterone, and dihydrotestosterone (DHT), contribute to BPH development. For BPH patients, two main treatment options exist: 5-alpha reductase inhibitors and alpha-1-adrenergic receptor antagonists [ 6].
DHT, a highly active metabolite of testosterone that is synthesized by the prostate 5-alpha reductase enzyme, is known to be a major contributor to BPH pathology [ 7]. Hence, 5-alpha reductase inhibitors such as finasteride and dutasteride have been used to treat BPH [ 8]. Alpha-1-adrenergic receptors exist in the prostate, urethra, bladder, vascular tissues, and central nervous system [ 9]. Alpha-1-adrenergic receptors are found in approximately 69.3% of the normal prostate tissue. In contrast, they are present in up to 85% of prostate tissue in BPH [ 9]. Alpha-1-adrenergic receptor antagonists, such as alfuzosin, doxazosin, tamsulosin, and terazosin, have been used to reduce the symptoms of BPH [ 10]. However, these drugs can produce undesirable side effects including erectile dysfunction, loss of libido, dizziness, severe myopathy, and chest pain [ 11].
Recent studies have suggested that commonly used herbal agents may effectively treat BPH or inhibit the development of BPH. Previous reports have shown that Aframomum melegueta extract ameliorates BPH in Wistar rats [ 12]. The extract consists of alkaloids, flavonoids, saponins, tannins, cardiac glycosides, terpenoids, and steroids. Melandrium firmum extract was also found to be effective against BPH development; it was found to contain some sapogenins, saponin, flavonoids, and triterpenoids [ 13]. Moreover, Bae et al. [ 14] reported that the water extract of Korean red ginseng and 20(S)-Rg3 represses androgen receptor activity. Saponins and ginsenoids are the major constituents of P. ginseng extract [ 15].
Ginseng is the commonly known name of the root of P. ginseng, which is very widely used in traditional herbal medicine in East Asia. P. ginseng has been evaluated for various protective effects against degenerative and aging-related conditions, such as neurodegenerative diseases [ 16], diabetic nephropathy [ 17], osteoporosis [ 18], ischemia, and oxidative stress [ 19]. however, the efficacy of P. ginseng against BPH has not yet been studied. Hence, in this study, we evaluated the effect of P. ginseng on a testosterone-induced BPH rat model and investigated the underlying molecular mechanism.
MATERIALS AND METHODS
Preparation of the Panax ginseng C.A. Meyer ( P. ginseng)
P. ginseng is Korean ginseng. The sample was collected at the Department of Medicinal Crop Research (Eumsung, Korea) in September 2010. To obtain the water extract of ginseng, 100 g of ginseng root was added to 600 mL of distilled water, and extraction was performed by heating at 95℃. It was then filtered through muslin cloth and lyophilized. The resulting powder (yield, 32 g) was dissolved in distilled water and sequentially passed through 0.22-µm filters for sterilization.
Animals
Seven-week-old male Wistar rats (Central Lab Animal Inc., Seoul, Korea) with an average body weight of 250±10 g were used in this study. The animal room was maintained at 22±2℃ and at 40%-70% relative humidity with a 12-hour light/dark cycle. All experiments were carried out according to the protocols approved by the Animal Care Committee of the Animal Center at Kyung Hee University and in accordance with guidelines from the Korean National Health Institute of Health Animal Facility [KHUASP(SE)-13-024].
Induction of BPH and Treatments
The testes of the rats in the BPH and the P. ginseng groups were removed to exclude the influence of intrinsic testosterone. The spermatic cord and blood vessels were ligated with Silkam sutures 3/8-16 mm (B.Braun Surgical SA, Rubi, Spain) and resected. BPH was induced by subcutaneous injection of testosterone (20 mg/kg; Wako chemicals, Tokyo, Japan) for 4 weeks after castration. Rats were divided into 3 groups (each group, n=10): (1) control group, (2) testosterone-induced (subcutaneous) BPH group, and (3) P. ginseng treated group (200 mg/kg oral administration; Sigma-Aldrich, St. Louis, MO, USA). Based on previous studies, we treated rats with 200 mg/kg of P. ginseng [ 20, 21]. All materials were administered to the animals once daily for 4 weeks, and body weight was measured weekly. After 4 weeks, all animals were fasted overnight. Blood was collected in ethylenediaminetetraacetic acid tubes, placed on ice, and the serum was immediately separated and stored at -20℃. After the animals were sacrificed, the tissue of prostate was stored in formaldehyde solution for light microscopic observation. The rest of the prostate was stored at -70℃ for subsequent analysis.
Blood Collection and Biochemical Analysis
At the end of the experiment, the food was removed and experiments were performed between 9 AM and 12 PM. Blood samples were obtained from the heart of the rats at the end of the experiment. Blood samples were rapidly centrifuged at 3,000 × g for 15 minutes at 4℃, and serum was obtained and stored at -70℃ before analysis. Glucose, total protein, glutamic oxaloacetic transaminase (GOT), and glutamic pyruvic transaminase (GPT) levels were analyzed by Greenlab (Seoul, Korea).
Histopathological Examination
Fixed prostate tissue embedded in paraffin wax were cut into 5-µm-thick sections and stained with hematoxylin and eosin. The sections were mounted and cover slipped using mounting solution and then examined under a microscope. Prostate epithelial thickness was measured.
Immunohistochemistry
Immunostaining was performed on 4-µm-thick sections after deparaffinization. Microwave antigen retrieval was performed in citrate buffer, pH 6.0, for 10 minutes prior to peroxide quenching with 3% H2O2 in phosphate buffered saline (PBS) for 10 minutes. Sections were then washed in water and preblocked with normal goat or rabbit serum for 10 minutes. In the primary antibody reaction, slides were incubated with anti-epidermal growth factor receptor (EGFR) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) in a 1:200 dilution and then anti-BCL2 (Santa Cruz Biotechnology) in a 1:200 dilution overnight at 4℃. The sections were then incubated with biotinylated secondary antibodies (1:1,000) for 1 hour. Following a washing step with PBS, streptodavidin-horseradish peroxidase was applied. Finally, the sections were rinsed in PBS and developed with diaminobenzidine tetrahydrochloride substrate for 10 minutes. At least three random fields of each section were examined at ×100.
RNA Extraction and Reverse Transcriptase-Polymerase Chain Reaction
Total RNA was isolated from the prostate tissues of each mouse using Trizol (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's instructions. An aliquot of total RNA was reverse transcribed and amplified using MMuLV reverse transcriptase and Taq DNA polymerase (Promega, Madison, WI, USA). Primers for reverse transcription (reverse transcriptase-polymerase chain reaction, RT-PCR) analysis were designed. The sequences of the designed primers were as follows: alpha-1D adrenergic receptor (Adra1d), sense: 5'-TGGTATCTGTGGGACCGCTA-3' and antisense: 5'-CACGATCACTGCCATGGGTA-3'.
Statistical Analyses
All the values are expressed as the mean±standard error. Significant differences between the groups were statistically analyzed using one-way analysis of variance (ANOVA), followed by a nonparametric post hoc Tukey test. All P-values are two-tailed, and significance was set at P<0.05. All statistical analyses were performed using PASW Statistics ver. 18.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Effect of P. ginseng on Weight Change
Body weight change is shown in Table 1. Final body weight was measured after 30 days, and each group showed an increase compared to their initial body weight (control group, 69.4%; BPH group, 70.2%; P. ginseng group, 69.4%). However, differences in weight change between the groups were not significant (P>0.05).
Effect of P. ginseng on Glucose, Total Protein, GOT, and GPT Levels in Serum
The BPH group displayed lower glucose levels than the control group ( Table 2). The plasma glucose levels of the P. ginseng treatment group were affected, but the differences were not statistically significant (P>0.05). GOT and GPT levels in serum were not significantly different among groups. P. ginseng did not promote the activity of the serum toxicity marker enzymes GOT and GPT indicating that each group of rats had normally functioning livers. Total protein levels in the serum of the BPH group were slightly elevated compared to those of the control, untreated group. However, the differences among the groups were not significant (P>0.05). The total protein levels of the P. ginseng group were similar to those of the control group.
Effect of P. ginseng on the Prostate
Prostate weight, volume, and weight ratio are shown in Table 3. The prostate weight of the BPH group (1.30±0.05 g) was significantly higher than that of the control group (1.01±0.03 g; P<0.001), and the P. ginseng-treated group (0.97±0.06 g) displayed a significantly lower prostate weight than the BPH group (P<0.001).
The prostate volume of the BPH group (1.75±0.15 cm3) was significantly higher than that of the control group (0.97±0.11 cm3), and the P. ginseng-treated group (0.93±0.14 cm3) displayed a significantly lower prostate volume than the BPH group (P<0.001).
The prostate weight ratio of the BPH group (0.38±0.03 mg/100 g of body weight [BW]) was significantly higher than that of the control group (0.28±0.01 mg/100 g of BW), and the P. ginseng-treated group (0.27±0.02 mg/100 g of BW) displayed a significantly lower prostate weight ratio than the BPH group (P<0.001).
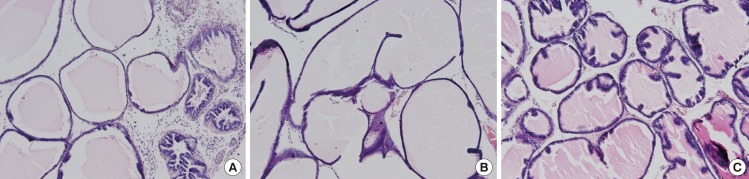
Effect of P. ginseng on the Histopathology of the Prostate Tissue
As shown in Fig. 1, the histoarchitecture of the prostate tissue in the BPH group was disrupted. The epithelial cell layer and stromal cell space in the BPH group were larger than those of the control group. Compared to the BPH group, the P. ginseng group showed reduced stromal cell hyperplasia and epithelial layer thickness.
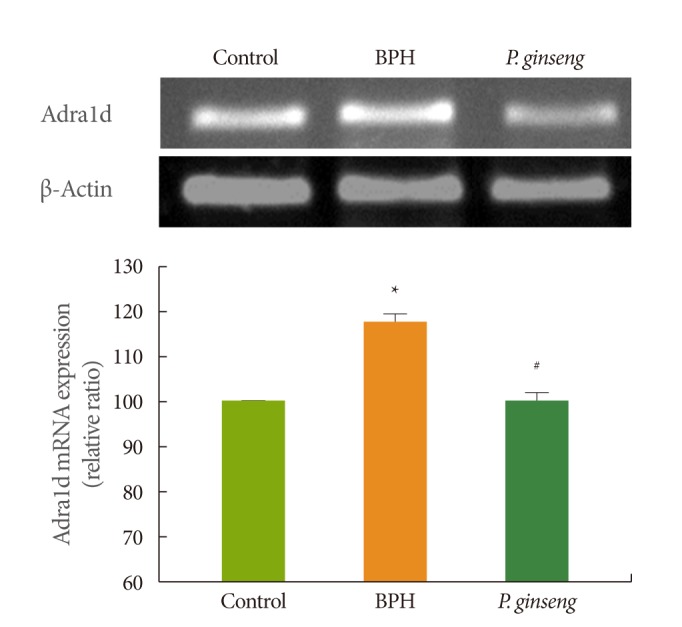
Effect of P. ginseng on Adra1d mRNA Expression in Prostate Tissue
At 4 weeks, the BPH group displayed high Adra1d mRNA expression levels compared to the control group ( Fig. 2). At 4 weeks, the P. ginseng group displayed significantly lower (P<0.05) Adra1d mRNA expression than the BPH group.
Effect of P. ginseng on the Expression of EGFR and BCL2 in Prostate Tissue
As shown in Fig. 3, the BPH group displayed higher EGFR and B-cell CLL/lymphoma 2 (BCL2) expression in prostate tissues than in the control group. EGFR and BCL2 expression in the P. ginseng group was lower than that in the BPH group (P<0.05).
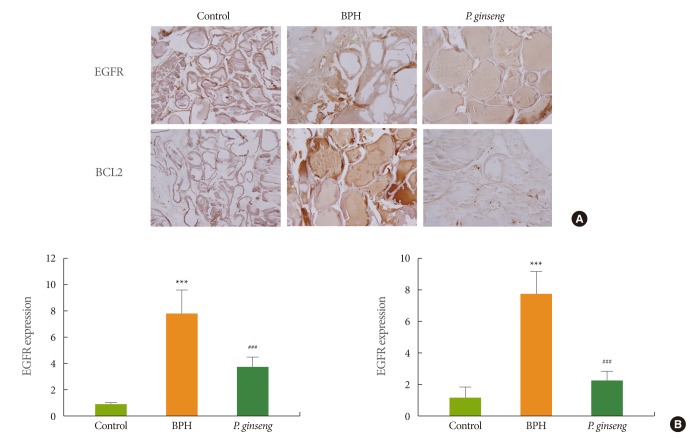
The expressions of epidermal growth factor receptor (EGFR) and B-cell CLL/lymphoma 2 (BCL2). (A) Immunohistochemistry of prostate tissue from the rats (×10). (B) The relative ratio of EGFR and BCL2 expression. BPH, benign prostatic hyperplasia; P. ginseng, Panax ginseng. ***P<0.001 compared with control group. ###P<0.001 compared with BPH group.
DISCUSSION
P. ginseng has been used as a herbal medicine to treat and prevent various diseases for more than 4,000 years. However, to our knowledge, no study has evaluated its inhibitory effect on BPH and the underlying mechanism. In this study, we observed the inhibitory effect of P. ginseng on BPH, investigated whether P. ginseng influences the alpha-1-adrenergic receptor and the development of BPH in a BPH rat model, and attempted to evaluate the inhibitory effect of P. ginseng on prostate hypertrophy.
Alpha-1-adrenergic receptors play important roles in the regulation of prostatic smooth muscle. Thus, they are critical regulators of LUTS and the development of BPH [ 22, 23]. These receptors have been classified into three subtypes, that is, Adra1a, Adra1b, and Adra1d, and have been observed in various tissues, such as the prostate, urethra, bladder, and ureter. These receptor subtypes are differentially localized in human prostate tissue. No difference was observed between the expression of Adra1a and Adra1b in the prostate tissue of control rats and BPH rats [ 24]. Rats with BPH showed significantly higher Adra1d expression than normal rats. It has been suggested that Adra1d may play a significant role in prostate growth in BPH [ 24]. There is also evidence that Adra1d is related to mitogen activated kinase-like protein, which mediates cell growth [ 25]. The mechanism underlying the effect of P. ginseng on adrenergic receptors has not yet been studied.
In this study, we observed increased Adra1d expression in the prostate tissue of BPH, and oral administration of P. ginseng significantly inhibited increase in Adra1d expression. P. ginseng treatment effectively reduced prostate volume compared to that seen for the BPH rats. In addition, we observed that the action of P. ginseng is associated with decreased EGFR and BCL2 expression in prostate tissues. EGFR and BCL2 expression was reduced by P. ginseng, which is in accordance with the inhibition of BPH development.
Alpha-1-adrenergic receptors also regulate growth and proliferation in various cells. Testosterone or DHT binds to nuclear androgen receptors on the surface of stromal and epithelial cells. It promotes the transcription of growth factors, including epidermal growth factor (EGF) [ 26, 27]. EGFR is the cell surface receptor for members of the EGF family. It regulates diverse biological processes in cell proliferation, differentiation, survival, and apoptosis [ 28]. Harper et al. [ 29] reported that increased EGFR have also been associated with the loss of growth control in BPH. Abnormal apoptosis is a known factor in the etiology of BPH. BCL2 is present in normal basal epithelial cells of the prostate gland and is expressed at significantly higher levels in BPH compared to those in normal prostate tissue. Iacopino et al. [ 30] also reported that BPH patients overexpress apoptosis-related genes, including BCL2. We found that P. ginseng decreased EGFR and BCL2 expression in the prostate tissues of BPH rat models. These observations indicate that P. ginseng may effectively inhibit increased EGFR and BCL2 expression and suppress BPH development.
Numerous studies have shown that ginseng contributes to EGFR and BCL2 signaling [ 31, 32, 33, 34]. Sathishkumar et al. [ 32] used P. ginseng to develop inhibitors for the EGFR tyrosine kinase domain. They showed that P. ginseng can be used to design new EGFR tyrosine kinase inhibitors. Fu et al. [ 34] showed that the total saponins in ginseng can protect against hepatic ischemia/reperfusion injury in experimental obstructive jaundice rats.
To summarize, orally administered P. ginseng extract was nontoxic and it inhibited the development of prostate hyperplasia and decreased prostate weight in testosterone-induced BPH rat models. We observed that these effects are associated with reduced Adra1d, EGFR, and BCL2 expression. These findings indicate that P. ginseng may be inhibiting the alpha1-adrenoceptor to suppress BPH development. Thus, P. ginseng may be useful in the treatment of BPH or in the prevention of prostatic hypertrophy. Further studies are required to confirm the involvement of other alpha-1-adrenergic receptors and EGF pathways in BPH.
Notes
This study was supported by grants from the "Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ009558)" Rural Development Administration, Republic of Korea.
All experiments were carried out according to the protocols approved by the Animal Care Committee of the Animal Center at Kyung Hee University and in accordance with guidelines from the Korean National Health Institute of Health Animal Facility [KHUASP(SE)-13-024].
No potential conflict of interest relevant to this article was reported