Bladder Pain Syndrome Treated with Triple Therapy with Gabapentin, Amitriptyline, and a Nonsteroidal Anti-Inflammatory Drug
Article information
Abstract
Purpose
Bladder pain syndrome is a chronic disease that manifests as bladder pain, frequency, nocturia, and urgency. Gabapentin, amitriptyline, and nonsteroidal anti-inflammatory drugs are efficacious treatments for bladder pain syndrome. Here, we assessed the effect of triple therapy with these drugs in women with bladder pain syndrome.
Methods
Between May 2007 and May 2010, we conducted a prospective nonrandomized study on 74 patients with bladder pain syndrome. Of these patients, 38 (11 men and 27 women; mean age, 55.9 years; range, 25 to 77 years; mean follow-up, 12.6 months) were administered the interstitial cystitis (IC) symptom scales (O'Leary-Sant Symptom Index) and visual analog scale (VAS) 1, 3, and 6 months after treatment to assess the efficacy of triple therapy.
Results
The pretreatment O'Leary-Sant IC symptom score was 11.7, and the post-treatment scores were 4.4, 3.8, and 4.0 at 1, 3, and 6 months, respectively; the pretreatment problem index score was 10.5, and the post-treatment scores were 3.7, 2.7, and 2.9 at 1, 3, and 6 months, respectively. The pretreatment VAS score was 6.7, and the post-treatment scores were 1.8, 1.5, and 1.7 at 1, 3, and 6 months, respectively. The O'Leary-Sant IC symptom index and problem index and VAS scores improved considerably 1 month after treatment (P<0.05). However, the results at 1, 3, and 6 months after treatment were not significantly different (P>0.05).
Conclusions
Triple therapy was sufficiently effective in patients with bladder pain syndrome and caused no significant adverse effects. However, large-scale studies should be performed to verify our findings.
INTRODUCTION
Bladder pain syndrome/interstitial cystitis (BPS/IC) is defined as unpleasant sensations such as pain, pressure, and discomfort perceived in relation to the bladder and lower urinary tract, with symptoms lasting for 6 weeks or longer in the absence of infection or any other confirmed causes [1]. The prevalence of BPS/IC varies greatly by nation, race, and definition, but the occurrence rate is approximately 0.3%, with BPS/IC being 10 to 20% less frequent among men than women. In some population-based studies that used questionnaires, however, BPS/IC was 30 to 50 times more frequent [1,2].
Although various etiologies are suggested, no clear clinical or pathological cause of BPS/IC has been identified, and there is no certain diagnosis or treatment. It is important to reassure the patient that the symptoms, although bothersome, are not signs of a life-threatening disease. Patient education and empowerment is an important initial step in therapy. In the absence of disease progression, treatment should focus on reducing symptom severity. Some patients get better naturally and even heal completely within a few weeks or months. Those patients who experience persistent, unacceptable symptoms despite reassurance and conservative treatment are candidates for more aggressive modalities. These might include oral and/or intravesical therapy, neuromodulation, treatment with pain killers or narcotic analgesics, and surgical intervention [1,3].
In terms of drug treatments, tricyclic antidepressants, anticonvulsants, and analgesics (nonsteroidal anti-inflammatory drugs [NSAIDs]) have been used on their own or in combination. Amitriptyline is an effective tricyclic antidepressant that suppresses the reuptake of serotonin and noradrenalin at presynaptic nerve ends and the anticholinergic reaction of the central nervous system and peripheral nerves, which in turn leads to sedation due to an antihistamine reaction in the central nervous system. The analgesic effect occurs at a dose of 25 to 150 mg per day and shows a good response within 30 to 50% [3-5]. Gabapentin is a structural analogue of γ-aminobutyric acid; it was first introduced as an anticonvulsant in 1994 and has been effectively used in various chronic pain treatments and is especially good for treating neuropathic pains [6-8].
Although there are some reports on the usefulness of gabapentin, amitriptyline, and NSAIDs, there are limitations to using a single drug to effectively control and treat a patient's pain. Therefore, in this study, we aimed to understand the effects and influences of a triple therapy of gabapentin, amitriptyline, and an NSAID by using the O'Leary-Sant Symptom Index and visual analog scale (VAS).
MATERIALS AND METHODS
We reviewed the records of patients who visited the urology clinic at our hospital from May 2007 to May 2010 and were diagnosed with BPS. Among the 74 patients who were treated with an NSAID, amitriptyline, and gabapentin, 38 patients were administered the O'Leary-Sant Symptom Index and VAS. Previous or current intake of amitriptyline, NSAIDs, and gabapentin was considered an exclusion criterion for study enrollment. Patients were subsequently treated prospectively for 6 months with a self-titration protocol. They were instructed to take 600 mg etodolac micronized, 5 mg amitriptyline, and 300 mg gabapentin at bedtime. If they were not symptom-free after 2 weeks, they were instructed to increase the dose to 20 mg amitriptyline and 600 mg gabapentin and thereafter to 75 mg amitriptyline and 900 mg gabapentin (maximum allowed doses). If the patients experienced satisfactory relief from their symptoms, they were asked to maintain the individual lowest effective dose and to not increase the dose further.
In order to determine treatment effects, the O'Leary-Sant Symptom Index and VAS were administered before and 1, 3, and 6 months after treatment. The O'Leary-Sant Symptom Index was divided into the IC Symptom Index and the IC Problem Index. We used SPSS ver. 13.0 (SPSS Inc., Chicago, IL, USA) for statistical analysis, and P-values below 0.05 were deemed statistically significant.
RESULTS
The subjects were 11 males and 27 females, with an average age of 55.9 years (range, 25 to 77 years) (Table 1). All patients complained of pelvic pain, pressure, and discomfort for 6 months or longer (main symptom) along with at least one other urinary symptom such as persistent urge to void or urination frequency in the absence of infection or other identifiable causes. The IC symptom index score before treatment was 11.7, the IC problem index was 10.5, and the VAS score was 6.7; all scores were quite high. At 1 month after treatment, the IC symptom index score was 4.4, the IC problem index score was 3.7, and the VAS score was 1.8; i.e., the scores had improved. After 3 months of treatment, the scores decreased: the IC symptom index score was 3.8, the IC problem index score was 2.7, and the VAS score was 1.5. At 6 months, the scores were as follows: the IC symptom index score, 4.0; the IC problem index score, 2.9; and the VAS score, 1.7 (Fig. 1). Complications while taking medication were drowsiness (n=3) and dry mouth (n=5), but not serious enough to stop the treatment.
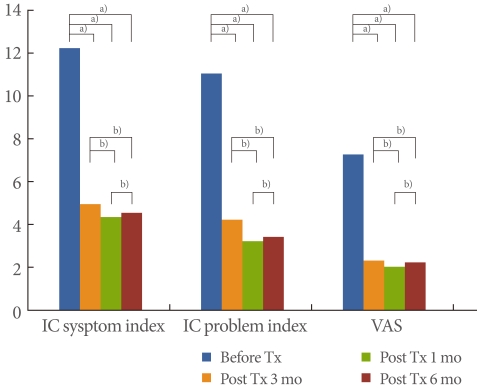
Changes in symptoms from baseline to 1, 3, and 6 months. IC, interstitial cystitis; VAS, visual analog scale; Tx, treatment. a)P<0.05. b)P<0.05 by Wilcoxon singed rank test.
Compared with before treatment, the IC symptom index score improved at 1, 3, and 6 months after the treatment by 62.4%, 67.5%, and 65.8%, respectively; the IC problem index score improved by 64.8%, 74.3%, and 72.4%, respectively; and the VAS score improved by 73.1%, 77.6%, and 74.6%, respectively. The improvements were statistically significant (P<0.05). Comparing the three post-treatment results, however, revealed that the scores obtained 1 and 3 months after treatment were slightly better but not statistically significant. The scores obtained 3 and 6 months after treatment indicated a slight deterioration in symptoms, but this deterioration was not statistically significant (P>0.05) (Fig. 1).
DISCUSSION
The clinical definition of BPS/IC should be expanded to include IC in cases with no tumor, infection, or any other identifiable cause of the symptoms. Chronic BPS/IC is defined as experiencing an unpleasant sensation in the bladder and lower urinary tract symptoms such as pain, pressure, or discomfort. However, there is no exact clinical etiology or diagnosis for IC as yet, and the treatments are quite diverse, ranging from behavioral therapy to cystectomy [1,2].
Pain is defined as an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage. Pain can be categorized as physiologic pain of the normal state and pathophysiologic pain of the abnormal state. Physiologic pain represents the nociceptive pain that disappears as soon as the nociception input disappears. Pathophysiologic pain continuously occurs, even after the nociception is gone or the tissue damage is repaired and can be further divided into inflammatory pain and neuropathic pain; it causes spontaneous pain, evoked pain, etc. Pathophysiologic pain is not a warning sign, but a pathological condition that harms the body, particularly if the inflammation or damage occurs around or in the nerve tissues. Sensitization of the primary afferent sensory fibers by mediators of the pain response results in greater, more frequent transmission of action potentials to nociceptive neurons than in 'normal' pain responses, and central sensitization can also cause an individual to perceive greater and more prolonged pain [6,7].
NSAIDs have anti-inflammatory, analgesic, and antipyretic effects. Because all NSAIDs and COX-2-suppressing drugs have equal effects on reducing pain, they have been used to treat chronic pain syndrome. The most serious and common complication of such NSAIDs is gastrointestinal disturbance [9].
The effects of anticonvulsants in pain treatment were first reported in the 1960s, and the anticonvulsant gabapentin was confirmed to reduce neuropathic pain. It is used to treat diabetic neuropathy, trigeminal neuralgia, pain due to cancer or multiple sclerosis, dyspareunia, and BPS [10-14]. Clinically, an anticonvulsant is effective for treating lancinating or burning pain, which is a type of chronic neuropathic pain. Gabapentinoids act as neuromodulators by selectively binding to the α2-δ-subunit protein of calcium channels in various regions of the brain and the superficial dorsal horn of the spinal cord. They also have a peripheral analgesic action [10-14].
Tricyclic antidepressants are used to treat various pain syndromes and cause effects such as increased pain tolerance, recovery of normal sleep, and decrease in depression symptoms [15]. They can be effective against chronic pain by directly suppressing the nervous mechanisms underlying pain or by alleviating depression symptoms caused by the ability to accept pain or experience pain. They control the activation and suppression of peripheral neurons or modulate the neuronal inhibitory or stimulatory pathways in the spine or supraspinal segments. Such mechanisms alleviate pain symptoms by suppressing acetylcholine, histamine, and the H1 receptor and by inhibiting the reuptake of released serotonin and norepinephrine [16]. Also, antidepressants are reported to be generally safe drugs with bearable side effects and low withdrawal symptoms in most studies [17,18].
Chronic pain causes more stress for a depressed patient, aggravating the condition, whereas anxiety or depression may strengthen the pain. Depression may influence nociception by reducing the activity of powerful descending inhibitory pathways that emanate from the brainstem. Serotonergic nuclei such as the nucleus raphae magnus of the medulla and various catecholamine nuclei located in the pons descend into the spinal cord via the dorsal funiculus. The activity of this pathway inhibits nociception at the level of the dorsal horn [19-21].
In BPS/IC patients, the independent application of NSAIDs, amitriptyline, and gabapentin was found to show about 30 to 50% efficacy in most reports, indicating that treatment with individual drugs has limited effects, and when a large dose is used, severe adverse effects may occur. To overcome the limitations of single-drug treatment, we devised a multimodal therapy with minimal amounts of an NSAID, etravil, and gabapentin.
Our triple therapy comprised minimal doses of the drugs, and we then compared scores before and after the treatment. The IC symptom index and IC problem index scores at 1, 3, and 6 months after treatment showed a 60 to 70% improvement, and the VAS score improved more than 70%. These improvements were statistically significant. After 1 month of treatment, however, the reduction in symptoms and pain were no longer significant. Therefore, we concluded that it is desirable to maintain low doses of these drugs to control the symptoms and pain after 1 month. If the pain goes away, the drug treatment should be stopped and the patient should be monitored carefully. When standardizing treatment efficacy, the level of pain and symptom scores during treatment will be important in BPS/IC research.
Our study had a few limitations. First, the number of patients was small, and the follow-up period was short. Such issues can be resolved by conducting a prospective study with more patients and lengthening the follow-up period. Second, in this study, because we evaluated the level of pain based on the memory of the patient, the responses were prone to recollection bias error. It is probable, however, that BPS/IC patients most sensitive to pain would have expressed the level of pain they experienced relatively better than other discomforts. Third, there was no control group. This study used the research model in which the pretreatment pain level of the patient was the control because it is ethically problematic to administer a placebo to patients with severe pain.
In determining the treatment effects for BPS/IC, the symptoms of the patient are used as an important index. We used the O'Leary-Sant Symptom Index and VAS scores before and after administering our triple therapy of gabapentin, amitriptyline, and an NSAID. The observed improvements were statistically significant, leading us to believe that the treatment was clinically helpful. We believe that this research objectively determined the effects of the triple treatment and the status of pain in BPS/IC patients. However, prospective research that supplements the limitations and issues of this study should be conducted.
ACKNOWLEDGEMENTS
This work was supported by Wonkwang University in 2010.
Notes
No potential conflict of interest relevant to this article was reported.