The In Vivo Effect of Ytterbium-Doped Fiber Laser on Rat Buccal Mucosa as a Simulation of Its Effect on the Urinary Tract: A Preclinical Histopathological Evaluation
Article information
Abstract
Purpose
The aim of this study was to perform a histological analysis of the effect of a ytterbium-doped fiber (YDF) laser on oral buccal mucosa tissue in vivo to simulate its effect on the mucosa of the lower urinary tract.
Methods
A total of 90 8-week-old Sprague-Dawley rats were anesthetized with urethrane (1.2 g/kg intraperitoneally). A prespecified inner buccal mucosal site was irradiated with a YDF master-oscillator power amplifier (MOPA) system for 60 seconds, with output power settings of 0.5, 1, and 2 W, respectively, in 3 treatment groups. Specimens of irradiated tissue were harvested at 2 hours, 24 hours, 2 weeks, and 4 weeks after irradiation. The tissue specimens were stained with hematoxylin and eosin for histological analysis.
Results
In the group treated with 0.5 W, basal cell elongation and vacuolization were observed at 2 hours and 24 hours after treatment, respectively. No evident injury was observed after 2 or 4 weeks. The group treated with 1 W presented partial basal layer separation, and even complete epidermal ablation, within 2 hours. At 24 hours after laser treatment, new capillaries on an edematous background of fibroblasts and myofibroblasts, as well as profuse infiltration of the neutrophils to the basal layer, were observed. Collagen deposition and reepithelization were observed in specimens taken 2 weeks and 4 weeks after treatment. The group treated with 2 W presented bigger and deeper injuries at 2 hours after irradiation. Meanwhile, subepidermal bullae with full-thickness epidermal necrosis and underlying inflammatory infiltrate were observed 24 hours after treatment. The presence of fibrous connective tissue and collagen deposition were observed 2 weeks and 4 weeks after the treatment.
Conclusions
To our knowledge, this is the first report regarding the effect of a YDF laser on living tissue. Our study demonstrated that the typical histological findings of the tissue reaction to the YDF MOPA apparatus were very similar to those associated with thermal injuries. The extent and degree of tissue damage increased proportionally to the output power.
INTRODUCTION
Due to their low invasiveness and high precision, lasers have become widespread in most medical disciplines during the last half century. In urology, several laser sources have become widely used, especially in the treatment of benign prostatic hyperplasia and urinary stones. Laser technology will evolve continuously, and newer lasers will become available in the future to improve the efficacy of both treatment and diagnosis.
Various types of lasers with wavelengths ranging from 100 to 10,000 nm (from ultraviolet to midinfrared) have been used in medical, industrial, communications, and military applications. High-power fiber lasers utilize advanced technologies to combine active optical fibers with semiconductor diodes. However, relatively few safety studies regarding tissue damage from continuous-wave exposures at these wavelengths can be found in the literature [1]. In particular, ytterbium-doped fiber (YDF) lasers, which have allowed great advances in industrial settings due to their high power, robust operation, and convenient fiber delivery, have remained underexplored. The fiber beam delivery system allows high flexibility in accessing difficult-to-reach areas, and Ytterbium-doped gain media have a low quantum defect and high output efficiency, which keeps costs low. These characteristics are favorable for clinical applications.
In this study, we developed a YDF master-oscillator power amplifier (MOPA) system with a wavelength of 1,050 nm, and evaluated the potential of this system for urological applications, including both diagnostic and therapeutic purposes. The aim of the present study was to investigate the effect of the YDF laser on buccal mucosal tissue in vivo. To the best of our knowledge, no information has been previously published regarding the response of living tissue to YDF lasers.
MATERIALS AND METHODS
Laser System
We developed in-house a prototype YDF MOPA system in order to validate its use in urological applications (Fig. 1). The prototype device consisted of a base station and a treatment hand piece. The base station housed the seed laser module and 3-stage YDF amplifiers. The seed laser module consisted of a seed laser diode with an operating wavelength of 1,050 nm, and a laser diode driver (NOTICE, Anyang, Korea) controlled by a computer. By using this laser diode driver, the repetition rate, average power, and pulse width of the seed laser could be tuned to the desired value. The seed signal was then amplified through 3 stages of YDF amplifiers. The average power of the system reached up to 3.4 W. The peak power of the laser pulse was determined by changing the repetition rate of the seed laser. The output of this system was connected to the treatment hand piece via a single-mode fiber [2,3]. The hand piece consisted of a focusing system that focused the laser beam on a focal spot (5 μm) within the target area. The settings for this study were: power of 0.5, 1, or 2 W, a repetition rate of 5 MHz, a pulse duration of 2 nanoseconds, and an exposure time of 60 seconds.
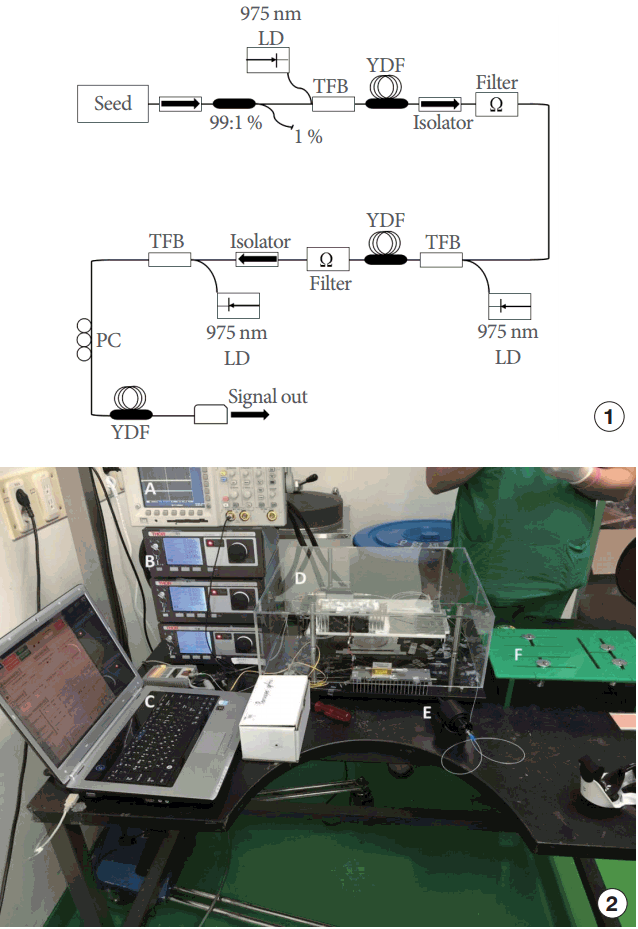
Schematic view of the 3-stage YDF MOPA system: (1) schematic plot, (2) pictures of real products. (A) Oscilloscope (TDS3032B, Tektronix, Beaverton, OR, USA). (B) Pump laser diodes (LDC 4020, Thorlabs, Newton, NJ, USA). (C) Laptop seed laser driver (SLDD401P, NOTICE, Anyang, Korea). (D) Three-stage YDF MOPA system (made in-house). (E) Focuser tip (focal length, 47 mm; spot size, 5 μm). YDF MOPA, ytterbium-doped fiber master-oscillator power amplifier; TFB, tapered fiber bundle; LD, laser diode.
Laser Treatment
A total of 90 male Sprague-Dawley rats weighing 250–300 g were studied using a protocol approved by the Institutional Animal Care and Use Committee (IACUC) of Seoul National University Hospital. The animals were housed in the vivarium, fed commercial rat chow and water ad libitum, and were then divided into 5 groups. Each group contained 5 animals. The laser settings were determined based on our unpublished previous pilot studies, which included experiments using fresh ex vivo chicken intestine with various energy and pulse repetition rate settings. Instead of conducting an experiment using urethral tissue in vivo, we decided to use buccal mucosa in this preliminary study. All animals were anesthetized using urethane intraperitoneally (1.5 g/kg). Of the 90 rats, 75 rats were treated with the YDF MOPA system at 510 mJ/cm2, using a 2-nanosecond pulse width at 5 MHz with a 5-μm spot size. The other 15 rats served as controls. The animals were sacrificed after laser application at 1 of the 3 different power settings (0.5, 1, or 2 W) and at 4 time points (2 hours, 24 hours, 2 weeks, or 4 weeks). To assess the tissue responses, the histological findings were analyzed using the ImageJ software (https://imagej.nih.gov/ij/index.html) [4].
Histopathological Analysis
For the histological analysis, sections were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned at 5 μm in serial horizontal sections, stained with hematoxylin and eosin (H&E) as previously described [5], and imaged using microscopy (Leica Microsystems, Wetzlar, Germany) at a magnification of ×200. For the purposes of statistical analysis, the degree of tissue response in the epithelium was scored according to the system described by Dai et al. [6].
Statistical Analysis
All statistical analyses were performed using IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA). The significance of differences was analyzed using the Mann-Whitney U test for non-Gaussian distributions and the unpaired t-test for Gaussian distribution. All data are presented as mean±standard deviation. P-values<0.05 were considered to indicate statistical significance.
RESULTS
Gross Findings
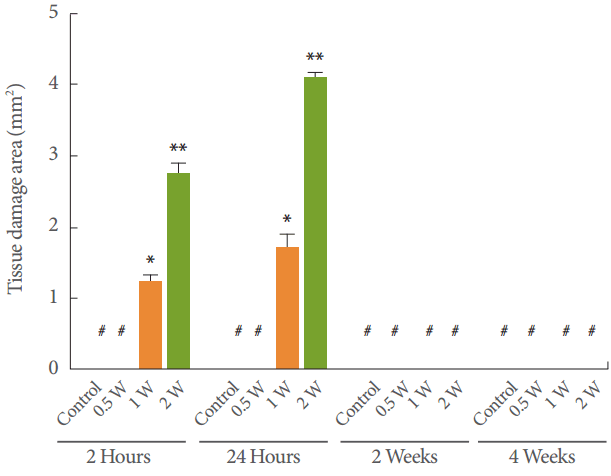
Each area of epithelial tissue was grossly observed immediately after the sites were irradiated. An in vivo skin specimen treated with the device is shown in Fig. 2. Laser-induced lesion patterns occurred after 60 seconds of irradiation at 1 and 2 W, and were accompanied by a burning odor. From 2 hours to 24 hours after irradiation, an obvious increase in lesion size was observed in the groups subjected to 1 W (P=0.01 and P=0.02, respectively) and 2 W (P<0.01) of irradiation compared to the control group. The surface was elevated with local hyperemia, but the center of the laser-applied lesions was often depressed. In contrast, no apparent lesions were observed in the treated area in the other groups. Edema was observed in the group that underwent irradiation with 2 W of power immediately after the treatment, became more significant at 24 hours, and resolved in approximately 2 weeks. No bleeding, blistering, scarring, or pigmentary changes were observed in the treated area during the post-treatment follow-up examinations.
Histological Findings
A lesion caused by treatment with the device, stained with H&E, is shown in Fig. 3. In the group treated with 0.5 W, basal cell elongation and vacuolization were observed at 2 and 24 hours after treatment (Fig. 3B, C). No evident injury was present after 2 or 4 weeks (Fig. 3D, E). The group treated with 1 W showed partial basal layer separation, and even complete epidermal ablation, within 2 hours (Fig. 3G). At 24 hours (Fig. 3H) after laser application, new capillaries on an edematous background of fibroblasts and myofibroblasts, as well as profuse infiltration of the neutrophils to the basal layer, were observed, suggesting a typical pattern of thermal injury. Collagen deposition and re-epithelization were observed in the specimens taken at 2 weeks (Fig. 3I) and 4 weeks (Fig. 3J) after the treatment. The group treated with 2 W presented bigger and deeper injuries at 2 hours (Fig. 3L) after treatment. Meanwhile, subepithelial bullae with full-thickness epidermal necrosis and underlying inflammatory infiltrate were observed 24 hours (Fig. 3M) after treatment. The presence of fibrous connective tissue and collagen deposition were observed at 2 weeks (Fig. 3N) and 4 weeks (Fig. 3O) after treatment. No zone of carbonized tissue was seen in any sample of histological specimens. The typical histological findings included basal cell elongation, nuclear shrinkage and cytoplasmic vacuolization, the formation of multiple basal lacunae, partial basal layer separation, and complete epithelial ablation. Irreversible injury to rat epithelial tissue was defined as the dosage value above which epidermal damage with a score ≥2 was noted. Fig. 4 shows the average scores of the tissue response in the epithelium following various YDF laser treatments in rats. The stars in Fig. 4 indicate zero scores of thermal injury to the epithelium 2 and 4 weeks after treatment with 0.5 W, and 4 weeks after treatment with 1 W or 2 W. In the group treated with 0.5 W, the average scores of thermal injury to the epidermis at 2 hours and 24 hours were 1.2 and 1.4, respectively. When the incident dosage was increased to 1 W, the average scores were 2.2, 2.6, and 0.2 after 2 hours, 24 hours, and 2 weeks, respectively (P=0.003, P=0.009, and P=0.752, respectively). When the incident dosage was further increased to 2 W, the average scores increased to 4.2 and 4.4 at 2 hours and 24 hours (P<0.01), but remained 0.2 at 2 weeks.
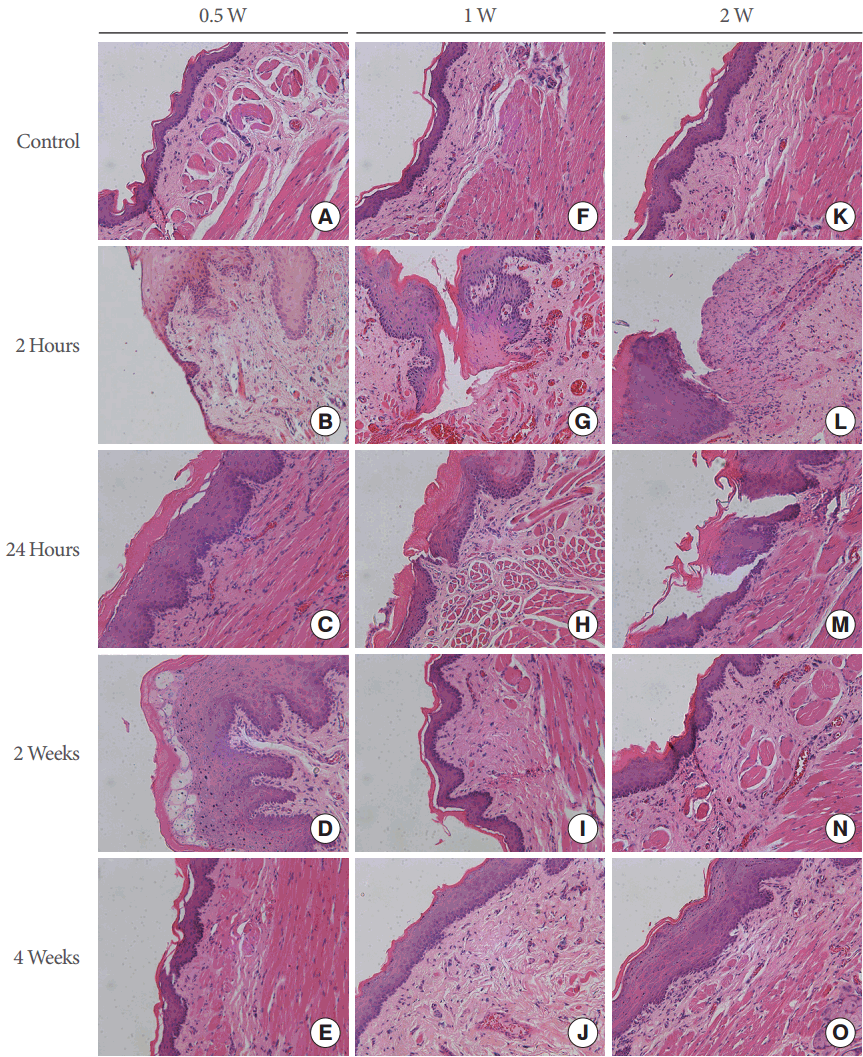
Histological observations of thermal injury following laser irradiation in hematoxylin and eosin-stained histological sections. The original magnification is ×200 in each photograph.
DISCUSSION
The use of lasers in urology is continuously evolving. Although some laser technologies have become established as standard modalities widely available to urologists in the last decade, others are still under investigation for applications in the future. The well-established holmium-doped yttrium aluminium garnet laser is a useful tool for tissue incision and ablation; it is widely used in lithotripsy for urinary stones [7], and in resection and enucleation for benign prostatic hyperplasia [8]. High-power potassium-titanyl-phosphate lasers are also widely used in urology, with many advantages, such as reduced postoperative hematuria leading to a shortened hospital stay and less postoperative catheter indwelling time [9,10]. However, the development of new laser technologies remains necessary since the current lasers used in clinical practice are not completely satisfactory.
YDF lasers are attractive because of their stability, high efficiency, and low production cost. Notable achievements have recently been made in the pulse energy and the pulse repetition rate of high-energy YDF lasers. Nedosekin et al. [11] suggested that a nanosecond-YDF laser operating at a high pulse repetition rate could be a promising optical source for time-resolved photoacoustic and photothermal cytometry, imaging, microscopy, and therapy. Sotsuka et al. [12] reported that YDF laser osteotomy demonstrated remarkable cutting efficiency in a rabbit model. Histological analyses showed little evidence of tissue damage in the muscle. Additionally, lased specimens exhibited no delayed healing response compared with what was obtained using the surgical saw. A recent study determined that automated bone ablation with an ytterbium-doped fiber laser created 3-dimensional target structures with limited thermal side-effects [13]. Above all, refinements to existing technology and new innovations will undoubtedly increase the role of lasers in the specialty. However, extensive knowledge of the negative effects of lasers on tissue is needed before they can be applied in humans.
Five types of laser interactions with tissues exist: photochemical interactions, photothermal interactions, photoablation, plasma-induced ablation, and photodisruption effects [14-16]. Usually, photothermal effects are mainly produced by infrared lasers, while ultraviolet radiation causes photochemical effects [14-16]. Among all known laser-tissue interactions, photothermal damage is the most important, since the rise in temperature caused by photothermal effects is the main factor that produces skin damage. In any laser-tissue interaction, the thermal effects depend on the local absorbed energy density at the target. In addition, the photothermal interaction between lasers and biological tissues has a range of thermal effects, such as coagulation, vaporization, and carbonization. The energy released by lasers also accumulates and diffuses in tissues, further extending its thermal effects and thermal damage. In this study, we aimed to examine the thresholds of laser damage to buccal mucosa for wavelengths near 1,050 nm, and to compare these thresholds with the current exposure limit definitions for a range of energy and exposure times.
Various tissues have been used as experimental models to assess thermal damage by lasers in rats. As the effect of lasers on tissues varies according to differences in water content and tissue density, we used the buccal mucosa for this preliminary study. This was mainly because the buccal mucosa has similar histological patterns to the urethral mucosa. Urologists have used buccal mucosal tissue as onlays, inlays, and tube-shaped substitutions in the surgical correction of urethral stricture [17]. The buccal mucosa is the preferred donor site for the repair of urethral stricture because it has a thick epithelium and abundant elastic fibers. Additionally, buccal mucosal tissue is easily accessible from the outside. Potential difficulties such as urinary retention or urinary tract infection can be avoided if buccal mucosal tissue is used.
An initial in vitro study performed to evaluate the YDF MOPA system showed that chicken intestinal mucosa remained intact when microscopic lesions were created (data not shown). The laser pulse energy and pulse repetition rate were chosen based on these pilot studies. Laser-induced thermal damage to mucosal tissue depends on the time-temperature response of tissues. This begins with the local absorption of the laser energy, which heats the tissue. The tissue may be affected by this heating through the denaturation of cellular proteins, leading to subsequent apoptosis or overt necrosis. At higher temperatures, thermal coagulation, collagen hyalinization, and changes in optical properties, such as increased scattering or birefringence, may be observed. The tissue response induced by the laser intensified significantly in the horizontal dimension with increased duration of the YDF MOPA system irradiation. Twenty-four days after laser irradiation, the epithelial response was more severe.
Histological findings such as basal cell elongation, nuclear shrinkage and cytoplasmic vacuolization, the formation of multiple basal lacunae, partial basal layer separation, and complete epithelial ablation were suggestive of thermal injuries [18]. No gross or histological injuries were observed in any of the areas treated with 0.5 W. The group treated with 1 W exhibited partial basal layer separation, and even complete epithelial ablation, within 2 hours. At 24 hours after laser application, new capillaries on an edematous background of fibroblasts and myofibroblasts, as well as profuse infiltration of the neutrophils to the basal layer, were observed. The group treated with 2 W presented bigger and deeper injuries 2 hours after irradiation. Meanwhile, subepidermal bullae with full-thickness epidermal necrosis and underlying inflammatory infiltrate were observed 24 hours after treatment. The stimulation of the inflammatory response may have been due in part to the photothermal effect of the laser. To assess the long-term tissue response, histological analyses were performed 2 weeks and 4 weeks after the treatment. Collagen deposition and reepithelization were observed in the specimens taken at 2 weeks and 4 weeks after treatment with 1 W. Moreover, the presence of fibrous connective tissue and collagen deposition were observed at 2 weeks and 4 weeks after irradiation with 2 W. No zone of carbonized tissue was seen in any sample of the histological specimens.
This study has several limitations. First, only a single laser was used, without any positive control, such as well-known lasers or common thermal burns, to compare thermal injury and wound healing over time. Second, temperature measurements were not performed after the laser treatment. Third, the mechanism of tissue recovery after local thermal damage is unknown. Despite these limitations, it is clear that when topically applied, a power of 0.5 W was safe in this model. Fourth, we could not measure the effect of the laser in a fluid-filled environment. The urinary tract is in constant contact with fluid; this is, therefore, a particularly important consideration, since the laser will be in contact with fluid in most urological applications. Further investigation is needed to elucidate this issue.
In conclusion, our study demonstrated that the typical histological findings of the tissue reaction to YDF laser application were very similar to those associated with thermal injuries. The extent and degree of tissue damage increased proportionally to the output power. These histological characteristics provide insights into the effect of YDF lasers on the mucosa of the urinary tract.
HIGHLIGHTS
- The present study is the first preclinical histopathological evaluation to show the effect of a YDF laser on living tissue as a simulation of its effect on the urinary tract.
- Our results demonstrated that typical histological finding of the tissue reaction to the YDF laser was very similar to those associated with thermal injuries.
Notes
Fund/Grant Support
This work was supported by the Interdisciplinary Research Initiatives Program from College of Engineering and College of Medicine, Seoul National University (grant number: 800-20140162).
Research Ethics
The study protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of Seoul National University Hospital.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.