High Estradiol Differentially Affects the Expression of the Glucose Transporter Type 4 in Pelvic Floor Muscles of Rats
Article information
Abstract
Purpose
To characterize the relationship between serum estradiol levels and the expression of glucose transporter type 4 (Glut4) in the pubococcygeus and iliococcygeus muscles in female rats.
Methods
The muscles were excised from virgin rats during the metestrus and proestrus stages of the estrous cycle, and from sham and ovariectomized rats implanted with empty or estradiol benzoate–filled capsules. The expression of estrogen receptors (ERs) was inspected in the muscles at metestrus and proestrus. Relative Glut4 expression, glycogen content, and serum glucose levels were measured. Appropriate statistical tests were done to identify significant differences (P≤0.05).
Results
The pubococcygeus and iliococcygeus muscles expressed ERα and ERβ. Glut4 expression and glycogen content in the pubococcygeus muscle were higher at proestrus than at metestrus. No significant changes were observed in the iliococcygeus muscle. In ovariectomized rats, the administration of estradiol benzoate increased Glut4 expression and glycogen content in the pubococcygeus muscle alone.
Conclusions
High serum estradiol levels increased Glut4 expression and glycogen content in the pubococcygeus muscle, but not in the iliococcygeus muscle.
• HIGHLIGHTS
- Glut4 expression and glycogen content in the pubococcygeus and iliococcygeus muscles are different in metestrus and proestrus.
- High estradiol levels, as a result of administering estradiol benzoate to ovariectomized rats, are related to greater Glut4 expression and glycogen content in the pubococcygeus muscle, but not in the iliococcygeus muscle.
- Both muscles express the α and β subtypes of estrogen receptors.
INTRODUCTION
Ovarian steroids appear to be relevant for the pelvic floor muscles (PFMs). Given the remarkable changes in estradiol levels across women’s lifespan, an understanding of estrogenic actions could be useful for managing some urogynecological dysfunctions. In this regard, intravaginal estrogen therapy can improve the results of PFMs training (PFMT), electrical stimulation, and biofeedback in post-menopausal women with stress urinary incontinence [1].
Female rats are commonly used to research the functional organization of the PFMs [2], such as the pubococcygeus and iliococcygeus muscles [3,4]. However, the hormonal milieu (i.e., relationship with the estrous cycle) has been consistently disregarded. Notwithstanding, a few reports have indicated intriguing relationships between estradiol levels and innervation, myofiber morphometry, and carbohydrate metabolism in the pubococcygeus [5-7].
Glucose transporter type 4 (Glut4) mediates up to 90% of the glucose uptake in adult skeletal muscles [8]. Insulin signaling promotes the translocation of Glut4-containing vesicles to the sarcolemma and facilitates Glut4 expression [9]. Estrogen modulates Glut4-dependent glucose uptake by 2 mechanisms: an acute mechanism, influencing the translocation to the sarcolemma, and a chronic mechanism, regulating the expression of Glut4 [10,11]. Once inside myofibers, glycolysis and glycogenesis are the main metabolic fates of glucose. Indeed, high Glut4 expression is directly related to glycogen content in skeletal muscles [12]. Moreover, estradiol levels influence Glut4 expression and glycogen content [12-14]. Importantly, glycogenesis predominates as a response to insulin stimulation with the overexpression of Glut4 in transgenic female rats, contrasting with male rats, in which glycolysis predominates [15].
Given the scarce information connecting estrogens with physiological processes in the PFMs, we evaluated herein the effect of estradiol on Glut4 expression and glycogen content in the pubococcygeus and iliococcygeus muscles of female rats. We first identified the expression of estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ) in both muscles because their activation modulates Glut4 expression in hindlimb muscles [10]. Second, a preliminary experiment was done to analyze variation in Glut4 expression and glycogen content at metestrus and proestrus, the stages of the estrus cycle in which serum estradiol levels are low and high, respectively [16]. Finally, we performed a second experiment to identify the direct influence of estradiol on Glut4 expression and glycogen content in sham and ovariectomized rats that were or were not administered estradiol benzoate (EB).
MATERIALS AND METHODS
Animals and Experimental Procedures
Wistar female rats were housed in individual polypropylene cages (37 cm×27 cm×16 cm) and maintained on a 12-hour light/dark cycle (lights on at 8:00 PM) at 20˚C±2˚C in the vivarium of the Centro Tlaxcala de Biología de la Conducta, Universidad Autónoma de Tlaxcala. They were daily provided with pellet food and had continuous access to water.
The estrous cycle of rats was monitored at the age of 3 months by examining vaginal smears [17]; samples were taken between 9:00 AM and 10:00 AM. ER expression in the pubococcygeus and iliococcygeus muscles was assessed at metestrus (M group; n=3) and proestrus (P group; n=3) in rats that were sacrificed around 10:30 AM and 11:00 AM, after 3 to 5 regular cycles (4 days each). For the first experiment, Glut4 expression and glycogen content were measured in the pubococcygeus and iliococcygeus muscles of 14 rats that were allocated to the M (n=7) and P groups (n=7). For the second experiment, 12 rats were ovariectomized bilaterally under ketamine (40 mg/kg, intraperitoneally [i.p.]) and xylazine (5 mg/kg, i.p.) anesthesia following a protocol adapted from Olson and Bruce [18]; the other 6 rats were subjected to sham surgery (the Sh group). After 4 weeks, the ovariectomized rats were implanted with an empty capsule (the OVX group) or a capsule filled with EB (the OVX+EB group). The capsules were made with a Silastic tube (10 mm long, 3.18 mm O.D., 1.98 mm I.D.; Dow Corning Corporation, Midland, MI, USA) sealed with wooden plugs. Females in the Sh group were sacrificed 6 weeks after surgery, during the metestrus stage, while those in the OVX and OVX+EB groups were sacrificed 2 weeks after implantation surgery. In all cases, an overdose of sodium pentobarbital was used (120 mg/kg, i.p.).
Blood samples were collected by cardiac puncture and allowed to clot for obtaining sera by centrifugation (10,000 rpm for 15 minutes). Sera were stored at -80˚C until assayed. Bilateral pubococcygeus and iliococcygeus muscles were dissected as described elsewhere [19]. The left muscles were frozen immediately after excision and stored at -80˚C until assayed. The wet weight of the uterus was recorded to assess the efficacy of the ovariectomy and EB treatment. Body weight was also recorded before surgery, 4 weeks after bilateral ovariectomy, and 2 weeks after capsule implantation.
Antibodies
Immunopeptides were detected with the following primary antibodies: mouse monoclonal immunoglobulin G (IgG) anti-Glut4 (1:200, cat. sc-53566, Santa Cruz Biotechnology, Santa Cruz, CA, USA), mouse monoclonal IgG antiglyceraldehyde phosphate dehydrogenase (1:500, GAPDH, cat. NB300-328, Novus Biologicals, Littleton, CO, USA), mouse monoclonal IgG anti-ATPase subunit 5A (1:500, cat sc-58613, Santa Cruz Biotechnology), mouse monoclonal IgG anti-ERα (1:200, cat. MA3-310, Thermo Scientific, Waltham, MA, USA), and mouse monoclonal IgG anti-ERβ (1:200, cat. MA1-23217, Thermo Scientific). The secondary antibody was goat anti-mouse IgG horseradish perioxidase (1:2,000, cat. sc-2005, Santa Cruz Biotechnology).
Western Blot
Nuclear protein extracts were prepared as described elsewhere [20] and used to identify ERα and ERβ expression in muscles. Total protein extracts were prepared to measure Glut4 relative expression as previously reported [21]. Briefly, equal amounts of protein (50 μg for nuclear and 100 μg for total protein extracts) were denatured in Laemmli’s sample buffer, resolved through 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and electro-blotted to nitrocellulose membranes. After staining with Ponceau’s Red (0.3% in acetic acid 1%, Amresco, Solon, OH, USA), the membranes were soaked in phosphate-buffered saline (PBS), blocked with 5% dried skim milk diluted in PBS, and incubated, first with the primary antibody (overnight at 4˚C), and then with the secondary antibody (2 hours at room temperature); antibodies were diluted with 1% dried skim milk in PBS. Immunoreactive polypeptides were detected using a chemiluminescence kit (West Pico Signal, Thermo Scientific) and exposed to a chemiluminescent-signal analyzer (MyECL, Thermo Scientific). Once finished, membranes were stripped with 100mM glycine (pH, 2.3) and 1% SDS, soaked in PBS, blocked with 5% dried skim milk diluted in PBS, and incubated with mouse monoclonal anti-GAPDH (for estimations in the M and P stages) or anti-ATPase subunit 5A (for the Sh, OVX, and OVX+EB groups) overnight at 4˚C; the GAPDH and ATPase bands were revealed as described above. The expression of Glut4 was measured by densitometry and normalized against that of GAPDH or ATPase expression per muscle and indicated as arbitrary units. ImageJ software (National Institutes of Health, Bethesda, MD, USA) was used for the densitometric analysis.
Serum Glucose and Muscle Glycogen Levels
The concentration of serum glucose was measured with a glucose-oxidase-based kit following the manufacturer’s instructions (Stanbio Glucose LiquiColor Oxidase, Ref. 1071, Stanbio Laboratory, Boerne, TX, USA). Serum samples were thawed, mixed with glucose assay buffer (1:100, v/v), and incubated for 10 minutes at room temperature. Absorbance was measured at the wavelength of 492 nm. Each sample was measured by duplicate and reported as mmol/L. Muscle glycogen content was measured by adapting a protocol reported by other authors [22]. Briefly, a portion of each muscle was homogenized in heated 2 M HCl (acid hydrolysis of glycogen chains releasing glucose molecules) or heated 2 M NaOH (basic hydrolysis, no glucose release). Samples were heated in boiling water for 1 hour and centrifuged at 10,000 rpm for 5 minutes at room temperature. The supernatant containing the product of hydrolysis was measured using a colorimetric enzymatic method to measure glucose using the kit mentioned above (Stanbio Laboratory). The muscle glycogen content was calculated as the difference between glycogen chains and free glucose and expressed as millimoles of glycosyl units per gram of muscle wet weight.
Serum Estradiol Levels
Total serum steroids were extracted with diethyl ether (Sigma-Aldrich, St. Louis, MO, USA) as reported elsewhere [21]. Total serum estradiol levels were then measured with an enzyme immunoassay-based commercial kit following the manufacturer’s instructions (item 582251, Cayman Chemical, Ann Arbor, MI, USA). Each sample was measured in duplicate.
Statistical Analysis
All measured variables are presented as mean±standard error of the mean unless otherwise stated. The level of statistical significance was set at P≤0.05. The unpaired 2-tailed Student t-test was used to identify significant differences between variables measured at metestrus and proestrus. For the Sh, OVX, and OVX+EB groups, significant differences regarding serum estradiol levels (data were log-transformed for this statistical test), Glut4 expression, and glycogen content were detected using 1-way analysis of variance followed by the uncorrected Fisher least significant difference test. Due to technical difficulties, glycogen content in the iliococcygeus muscle was measured only in 4 rats in the Sh and OVX groups, and in 6 rats in the OVX+EB group. All statistical tests were done using the software Prism (GraphPad Software, La Jolla, CA, USA).
RESULTS
Estrogen Receptor Expression in the Pubococcygeus and Iliococcygeus Muscles
The presence of a band of -66 kDa revealed ERα expression in the pubococcygeus and iliococcygeus muscles at metestrus and proestrus (Fig. 1A). Similarly, a band of -55 kDa revealed ERβ expression in the pubococcygeus and iliococcygeus muscles of rats at metestrus and proestrus (Fig. 1B).
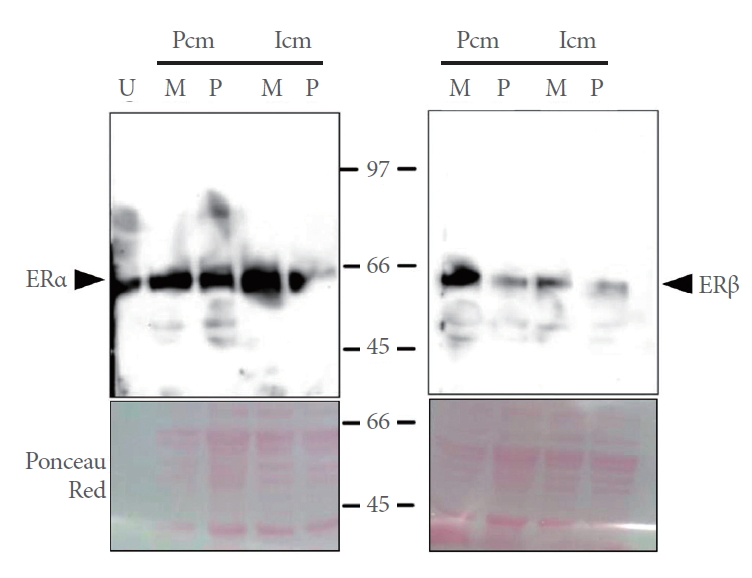
Expression of ERα and ERβ in the Pcm and Icm of rats at metestrus (M) and proestrus (P). Nuclear protein extracts from the Pcm and Icm were loaded. Expression of ERα was assessed in uterine extracts (U) as a positive control of ERα expression. Representative photographs of Ponceau’s Red-stained membranes are shown. ER, estrogen receptor; Pcm, pubococcygeus muscle; Icm, iliococcygeus muscle.
Experiment 1
Glut4 expression in the pubococcygeus and iliococcygeus muscles
Glut4 expression (-50 kDa) was observed in the pubococcygeus and iliococcygeus muscles (Fig. 2A). Glut4 expression in the pubococcygeus muscle at proestrus was significantly higher than at metestrus (P=0.015) (Fig. 2B, D). For the iliococcygeus muscle, Glut4 expression was not significantly different between both stages of the estrous cycle (P=0.739) (Fig. 2C, E).

Glut4 expression in the Pcm and Icm at metestrus (M) and proestrus (P). (A) Representative immunoblot showing Glut4 expression in the Pcm and Icm. Relative expression of Glut4 in the Pcm (B) and Icm (C) is shown as the quotient (arbitrary units [a.u.]) obtained by dividing the density of the Glut4 band by that of the glyceraldehyde phosphate dehydrogenase (GAPDH) band (D, E). Data are means±standard error of the mean (n=7 for each group). Two-tailed unpaired t-tests; *P<0.05. Glut4, glucose transporter 4; Pcm, pubococcygeus muscle; Icm, iliococcygeus muscle.
Glycogen content in the pubococcygeus and iliococcygeus muscles and serum glucose levels
The glycogen content of the pubococcygeus muscle at proestrus was significantly higher than at metestrus (9.8±3.7 vs. 0.96±0.28 mmol of glycosyl units per gram of muscle wet weight, n=7; P=0.035). In contrast, the glycogen content of the iliococcygeus at metestrus and proestrus was not significantly different (3±2.1 vs. 1.3±0.73 mmol of glycosyl units per gram of muscle wet weight, n=7; P=0.471). However, the concentration of serum glucose at proestrus (8.5±0.7 mmol/L, n=7) was significantly lower than at metestrus (12.1±1 mmol/L; t=2.943; P=0.012; n=7).
Experiment 2
Bodily measurements
The body weight at sacrifice was not significantly different (P=0.129) among the Sh (249±12 g), OVX (287.9±15.6 g), and OVX+EB (260.4±10 g) groups. However, 4 weeks after surgery, the body weight gain of the ovariectomized rats was significantly higher than that of the rats subjected to the sham surgery (P=0.003) (Fig. 3A). Two weeks after the capsule implantation, the body weight gain was significantly different among the Sh, OVX, and OVX+EB groups (P=0.008), and post hoc tests showed that the body weight gain in the OVX group was significantly higher than that in the Sh and OVX+EB groups (Fig. 3A). The normalized weight of the uterus (mg/g body weight) changed significantly among groups (P=0.0059), and post hoc tests indicated that a significant reduction only took place in the OVX group, but not in the Sh and OVX+EB groups (Fig. 3B). The concentration of serum estradiol was significantly different among groups (P<0.0001). Thus, the estradiol concentration significantly decreased in the OVX group in comparison with the Sh group, while a significant increase was found in the OVX+EB group in comparison with both the Sh and OVX groups (Fig. 3C).

Glut4 expression in the Pcm and Icm in the Sh, OVX, and OVX+EB groups. (A) Body weight gain of female rats at 4 and 6 weeks (2 weeks after capsule implantation, vertical dashed line) after bilateral ovariectomy. (B) Uterus wet weight at sacrifice day. (C) Serum estradiol levels. Representative immunoblots of Glut4 and ATPase 5A in Pcm (D) and Icm (E); the Icm samples were run in the same gel and a lane was removed for final presentation (note solid line at splice site) because it was not related to the present study. Relative expression of Glut4 in the Pcm (F) and Icm (G) is shown as the quotient (arbitrary units [a.u.]) obtained by dividing the density of the Glut4 band by that of the ATPase subunit 5A (ATPase 5A) band (D, E). Data are means±standard error of the mean. (n=6 for each group). *P<0.05. **P<0.01. ****P<0.0001. Glut4, glucose transporter 4; Pcm, pubococcygeus muscle; Icm, iliococcygeus muscle; Sh, sham; OVX, ovariectomized; EB, estradiol benzoate.
Glut4 expression in muscle and variation in serum estradiol levels
The expression of Glut4 in the pubococcygeus muscle was significantly different among the groups (P=0.003) (Fig. 3D, F). Glut4 expression was higher in the OVX+EB group than in the Sh and OVX groups, while no significant difference was detected between the Sh and OVX groups. In contrast, the expression of Glut4 in the iliococcygeus muscle did not significantly differ among the 3 groups (F=2.221, P=0.143) (Fig. 3E, G).
Muscle glycogen and variation in serum estradiol levels
The glycogen content of the pubococcygeous muscle was significantly different (P=0.046) among the Sh (1.2±0.4 mmol of glycosyl units per gram of muscle wet weight), OVX (0.96±0.3 mmol of glycosyl units per gram of muscle wet weight), and OVX+EB (2.2±0.3 mmol of glycosyl units per gram of muscle wet weight) groups, and post hoc tests indicated a significant increase in the glycogen content of the OVX+EB group compared with the Sh (P<0.05) and OVX groups (P<0.05). No significant differences were found between the Sh and OVX groups. In contrast, the glycogen content of the iliococcygeus muscle was not significantly different (P=0.298) among the Sh (0.8±0.5 mmol of glycosyl units per gram of muscle wet weight), OVX (0.13±0.04 mmol of glycosyl units per gram of muscle wet weight), and OVX+EB (0.44±0.13 mmol of glycosyl units per gram of muscle wet weight) groups. Otherwise, the serum glucose levels did not significantly vary among the groups (Sh: 9.5±0.7; OVX: 10.1±0.5; OVX+EB: 8.8±0.4 mmol/L; P=0.320).
DISCUSSION
Considering the positive modulation that high estradiol levels exert on Glut4 expression in hindlimb muscles [14], it seems reasonable to suggest that its rise at proestrus could lead to augmented Glut4 expression in the pubococcygeus muscle. Nevertheless, testosterone and progesterone levels also increase at proestrus [16,23]. In this regard, the former hormone could promote augmented Glut4 expression, while the latter could reduce it [14,24]. Thus, differences in the relative levels of ovarian steroids may influence Glut4 expression in the pubococcygeus muscle. Although both the pubococcygeus and iliococcygeus muscles express ERα and ERβ, the fact that the iliococcygeus muscle seems to be unresponsive to variation in steroid hormones at metestrus and proestrus may be thus related to the opposing actions initiated by testosterone, progesterone, and estradiol. In any case, further experiments should evaluate the role of the expression of androgen and progesterone receptors in Glut4 expression in the muscles evaluated herein. Such an analysis should include the ER subtypes. Furthermore, serum insulin levels reach their highest levels at proestrus and their lowest levels at diestrus [23]. Although we did not measure insulin levels herein, it could reasonably be expected that the increase in serum insulin levels at proestrus would increase Glut4 expression similarly in the pubococcygeus and iliococcygeus muscles, but this was not the case.
Bilateral ovariectomy was effective in reducing circulating estradiol, as confirmed by the body weight gain and normalized uterus weight. In contrast, ovariectomized rats implanted with EB capsules had higher estradiol levels. Thus, the present data support the proposal that Glut4 expression in the iliococcygeus muscle is not related to serum estradiol levels because this remained unaltered in the OVX and OVX+EB groups. Such a finding was clearly different from that observed in the pubococcygeus muscle, in which high serum estradiol levels were associated with augmented Glut4 expression. Certainly, muscle-specific factors, including MEF2, MyoD, and GEF, directly regulate the transcription of the SLC2A4 gene, which codes for Glut4, while estradiol could act more indirectly [9]. The results herein and those of other studies support the notion that high estradiol levels at proestrus can upregulate Glut4 expression in the pubococcygeus muscle [14,25]. The mechanisms involved in the specific modulation of Glut4 expression in the pubococcygeus and iliococcygeus muscles should be further explored by analyzing transcriptional repression and/or epigenetics triggered by ER activation [9,25,26]. In this regard, it is important to consider that ERα activation promotes, while ERβ activation represses, Glut4 expression in hindlimb muscles [10]. Knowing the ERα-to-ERβ ratio would have been an excellent starting point for an analysis of this issue, but unfortunately we did not measure this ratio herein due to the insufficient number of rats and the primary intention of the study to identify ER expre ssion in the pubococcygeus and iliococcygeus muscles.
Whereas the glycogen content of the pubococcygeus muscle at proestrus was higher than at metestrus, there was no change in the glycogen content of the iliococcygeus between both stages of the estrous cycle. Some studies have demonstrated that higher Glut4 expression was associated with greater glycogen content in skeletal muscles [12,15], which may explain our findings about the pubococcygeus muscle. However, ovarian steroids can influence hindlimb muscle glycogen content, as supported by the fact that estradiol has a positive effect while progesterone has a counteracting negative effect [13,27]. Certainly, greater glycogen content could imply a higher rate of synthesis or a slower rate of degradation. In this regard, estradiol restores the glycogen content in the red quadriceps muscle after exercise in ovariectomized rats, while progesterone has no effect [14]. Hence, the present findings from ovariectomized rats treated with EB resemble the findings of high glycogen content in the pubococcygeus at proestrus, supporting the notion that estrogenic actions are involved. In clear contrast, no changes in glycogen content were seen in the iliococcygeus muscle.
The limitations of our present study include the fact that the rats were not fasting before the tissues were excised and that serum insulin levels were not measured. Both of these factors are relevant for understanding the metabolism of glucose in skeletal muscles. However, our data support the proposal that ovarian hormones, and particularly estradiol, may exert a complimentary role in modulating Glut4 expression and glycogen content in the pubococcygeus muscle, but not in the iliococcygeus muscle, as the extent to which circulating insulin levels would influence them is expected to be similar (i.e., higher insulin levels in nonfasting rats are associated with greater Glut4 expression in both muscles). The functional expression of insulin receptors and negative regulators of insulin signaling should therefore be approached for each muscle.
The present findings support the proposal that the pubococcygeus muscle is sensitive to changes in the hormonal milieu due to the estrous cycle. Insights from Glut4 expression and glycogen content in the pubococcygeus muscle point to the involvement of estrogenic actions that should be properly analyzed in further experiments. The apparent insensitivity shown by the iliococcygeus muscle also requires further evaluation, with an emphasis on the expression of particular ER subtypes and other steroid receptors (e.g., androgen and progesterone receptors). As different responses were observed regarding Glut4 expression in each muscle, and since Glut4 expression is upregulated by insulin and exercise in hindlimb muscles [7], the findings herein could be relevant for understanding the synergistic actions between PFMT and estrogen administration for strengthening the PFMs [1]. Overall, differences regarding Glut4 expression and glycogen content in the pubococcygeus and iliococcygeus muscles could be linked to ovarian steroid levels at proestrus and metestrus. By analyzing these variables in ovariectomized rats according to EB treatment, the findings herein suggest that high estradiol levels are related to greater Glut4 expression and glycogen content in the pubococcygeus muscle, but not in the iliococcygeus muscle, although both muscles express ERs.
Acknowledgements
The authors thank Laura García Rivera, Guadalupe Citlaly Hernández Hernández, and Shareth Y. Rodríguez Jaimes for the excellent technical assistance provided.
Notes
Grant/Fund Support
This study was funded by the Dirección General de Asuntos del Personal Académico (DGAPA-UNAM; IA203617) and the Consejo Nacional de Ciencia y Tecnología of México (CONACyT; 225126).
Research Ethics
The Institutional Committee for Care and Use of Laboratory Animals of the Instituto de Investigaciones Biomédicas of the Universidad Nacional Autónoma de México approved all experimental procedures involving animal subjects included in the present study (project ID number: 243).
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTION STATEMENT
· Full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis: CF
· Study concept and design: CF
· Acquisition of data: CRMA, HALG, JRCR
· Analysis and interpretation of data: CRMA, HALG, JRCR
· Drafting of the manuscript: CRMA, CF
· Critical revision of the manuscript for important intellectual content: RAJ, PP, CRE, MGM
· Statistical analysis: CRMA, CF
· Obtained funding: CF
· Administrative, technical, or material support: RAJ
· Study supervision: PP, CRE, MGM, CF
