Artificial Urinary Sphincter Cuff Size Predicts Outcome in Male Patients Treated for Stress Incontinence: Results of a Large Central European Multicenter Cohort Study
Article information
Abstract
Purpose
The aim was to study the correlation between cuff size and outcome after implantation of an AMS 800 artificial urinary sphincter.
Methods
A total of 473 male patients with an AMS 800 sphincter implanted between 2012 and 2014 were analyzed in a retrospective multicenter cohort study performed as part of the Central European Debates on Male Incontinence (DOMINO) Project.
Results
Single cuffs were implanted in 54.5% and double cuffs in 45.5% of the patients. The cuffs used had a median circumference of 4.5 cm. Within a median follow of 18 months, urethral erosion occurred in 12.8% of the cases and was associated significantly more often with small cuff sizes (P<0.001). Multivariate analysis showed that, apart from cuff size (P=0.03), prior irradiation (P<0.001) and the penoscrotal approach (P=0.036) were associated with an increased erosion rate. Continence rate tended to be highest with median cuff sizes (4–5.5 cm).
Conclusions
Apart from irradiation and the penoscrotal approach, small cuff size is a risk factor for urethral erosion. Results are best with cuff sizes of 4.5–5.5 cm.
INTRODUCTION
Stress urinary incontinence is a common complication even in modern laparoscopic and robotic-assisted radical prostatectomy. Twenty percent of the patients continue to have urine loss for more than a year [1]. Other common reasons for stress urinary incontinence are orthotopic neobladder, transurethral desobstruction and neurogenic diseases. Since 1987 the AMS 800 artificial sphincter has been the gold standard for treating moderate to severe male stress urinary incontinence. A common problem, however, is persistent or recurrent urinary incontinence in 14%–96% and urethral erosion in 8.5% of the cases [2]. The surgeon’s experience significantly reduces the complication rate; moreover, urethral exposure, precise cuff sizing and correct placement are decisive steps toward achieving patient satisfaction [3]. Cuffs are available in sizes of 3.5 to 9 cm and can be implanted as single or double cuffs (SC or DCs). Within the framework of the Debates on Male Incontinence (DOMINO) Project, 16 Central European hospitals that implant artificial sphincters took part in our retrospective multicenter cohort study investigating the influence of cuff size on outcome in patients with an AMS 800 artificial urinary sphincter (AUS).
MATERIALS AND METHODS
Our multicenter cohort study (DOMINO Project) included 473 male patients who had received an AMS 800 sphincter between 2012 and 2014. Ethics approval was obtained for the retrospective analysis. All data were recorded by external investigators. Analysis covered incontinence-triggering surgery or irradiation, incontinence surgery, and the position, number and location of cuffs. Endpoints were urethral erosion requiring revision, infection, and mechanical complications as well as incontinence, and the achievement of social continence (0–1 pad/24 hours).
Statistical analysis was done with IBM SPSS Statistics ver. 24.0 (IBM Co., Armonk, NY, USA). Contingency tables and Fisher exact test were used for evaluation. Kaplan-Meier analysis was conducted to estimate implant durability, and differences between the curves were calculated using the log-rank test. Regression analysis of multivariate binary data included cuff size and other pre-established factors influencing outcome: irradiation yes/no, number of cuffs (SC/DC), and cuff position (penoscrotal/perineal, PS/PN). P-values<0.05 were considered statistically significant.
RESULTS
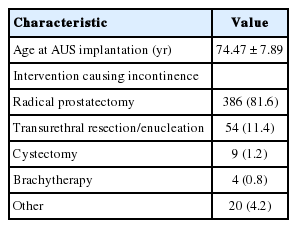
A total of 473 male patients with an AMS 800 were identified. They had a mean age of 74.5 years (standard deviation, 7.9 years) at the time of the incontinence-triggering event. Follow-up data were available for 424 patients (89.6%) and covered a median period of 18 months. The most common cause of stress incontinence was radical prostatectomy (n=386, 81.6%), followed by transurethral desobstruction (n=54, 11.4%). Implantation was mostly performed via a perineal approach (PN: n=359, 76% vs. PS: n=113, 24%). A SC was used in 258 patients (54.5%), a DC in the others (n=215, 45.5%). Cuffs size ranged from 3.5 to 9 cm and had a median circumference of 4.5 cm (mean value, 4.59 cm). Tables 1-3 outlines the distribution of cuff sizes as well as other patient and implant factors.
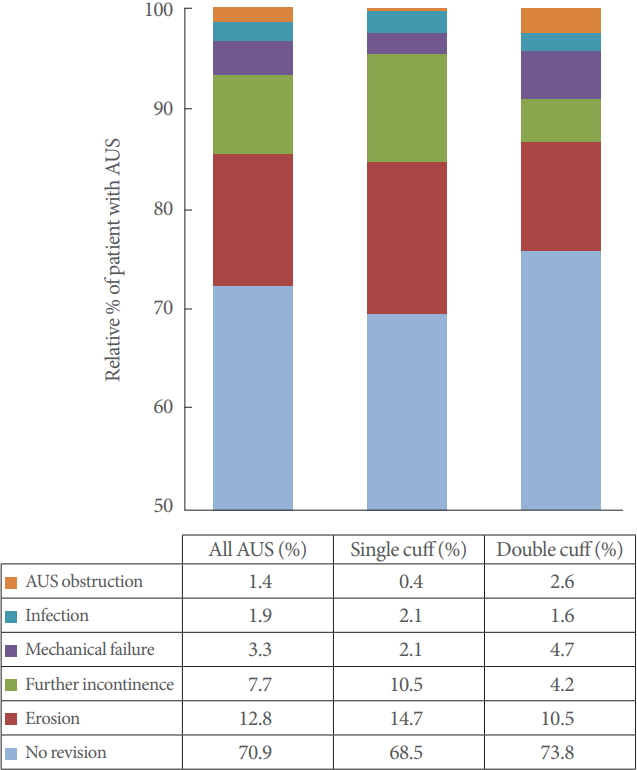
In our cohort, 125 patients (29.1%) required AUS revision. The most frequent reasons were urethral erosion (n=55, 12.8%) and persistent incontinence (n=33, 7.7%). Infection or mechanical malfunction was rare. Fig. 1 graphically depicts the individual subgroups. Transurethral resection or indwelling catheter led to erosion in 6 patients (1.4%).
Data on the postoperative continence situation could be obtained in 336 of 473 patients (71%). Seventy-seven males (22.9%) become continent (0 pads/day), social continence (0–1 pads/day) was achieved in 138 cases (41.1%), and some kind of improvement over the preoperative situation was seen in 210 (62.8%).
Cuff Size
The median and mean cuff sizes were 4.5 and 4.7 cm for perineal and 4.5 and 4.3 cm for penoscrotal implantation, respectively. The mean cuff size was 4.4 cm in irradiated and 4.7 cm in radiation-naïve patients.
Single Cuff
Cuff lengths ranged from 3.5 cm to 9 cm with a mean circumference of 4.73 cm for single cuffs. Revision rates are listed in Fig. 1. Patients were subdivided into groups to statistically analyze the correlation between outcome and cuff size (3.5 cm; 4 cm; 4.5 cm; 5–5.5 cm; ≥6 cm). The results are graphically depicted in Fig. 2.
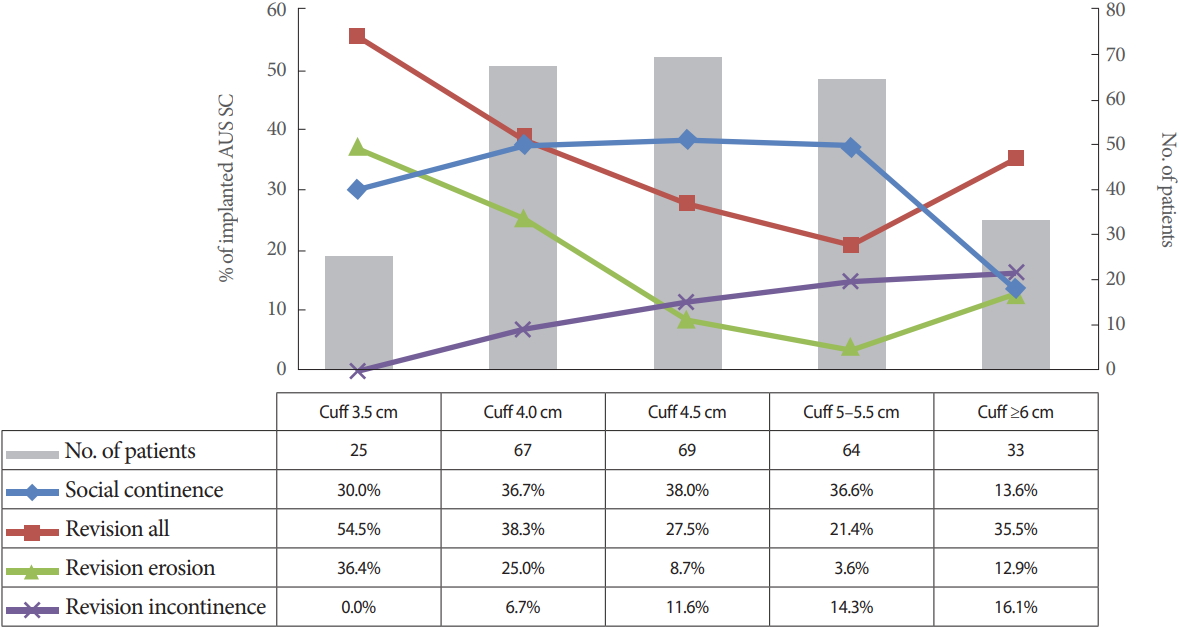
Most common reasons for revisions and social continence in relation to cuff size in patients with artificial urinary sphincter (AUS) and single cuff (SC).
Univariate analysis disclosed no significant correlation between cuff size and the explantation rate in general. Urethral erosion occurred significantly more often with small cuff sizes (P<0.001). Revision due to persistent incontinence was more common in patients with large cuff sizes (≥6 cm, 15.6%; 3.5 cm, 0%). However, this finding was not statistically significant.
Full continence and social continence were achieved mainly with median cuff sizes, though this finding was not statistically significant either (Fig. 2). Moreover, cuff size did not correlate significantly with infection, revision for cuff obstruction with voiding dysfunction, or mechanical complications.
Kaplan-Meier analysis was done for cuff-related urethral erosion (Fig. 3). The 18-month implant survival rate was lowest for 3.5-cm cuffs and highest for 5–5.5 cm cuffs (18-month cuff survival: 3.5 cm, 60.2%; 4.0 cm, 68.9%; 4.5 cm, 88.7%; 5–5.5 cm, 96.1%; ≥6 cm, 83.6%; log rank P=0.001).
Double Cuff
Evaluation of the smaller of the 2 cuffs used disclosed a mean cuff size of 4.43 cm. Cuff sizes did not vary widely in DC; the 4.5 cm cuff was used most often (n=139, 64.7%) No significant correlation was found between cuff size and outcome for the revision rate in general (P=0.28) or for revisions because of incontinence (P=0.76), infection (P=0.68), cuff obstruction (P=0.41), or mechanical complications (P=0.33). The revision rate for erosion tended to be lower with 4 or 4.5 cm cuffs (P=0.06).
Full continence and social continence did not depend on the smaller of the 2 selected cuff sizes. Kaplan-Meier analysis was again done for cuff-related urethral erosion (Fig. 4). The 18-month implant survival rate was highest for 4.5 cm cuffs (18-month cuff survival: 3.5 cm, 0%; 4.0 cm, 90.3%; 4.5 cm, 94.1%; 5–5.5 cm, 83.3%; log-rank P=0.014).
Multivariate Analysis
Since urethral erosion occurred significantly more often with small single cuffs, multivariate analysis included the following variables: cuff size, number of cuffs SC/DC, localization PS/PN, and irradiation yes/no. Urethral erosion did not correlate with SC/DC (P=0.84; confidence interval [95% CI], 0.5–2.3) but did correlate significantly with cuff size (P=0.03). In relation to the 3.5-cm cuff, the probability of erosion was lowest with the median cuff sizes (4.0 cm: hazard ratio [HR], 0.59; CI, 0.2–1.74; 4.5 cm: HR, 0.23; CI, 0.08–0.7; 5–5.5 cm: HR, 0.32; CI, 0.09–1.15; ≥6 cm: HR, 0.74; CI, 0.16–3.5). The penoscrotal approach led to urethral erosion significantly more often than the perineal approach (HR, 2.2; CI, 1.06–4.86; P=0.036). Irradiation was also associated with urethral erosion to a highly significant degree (HR, 3.6; CI, 1.92–6.7; P<0.001).
DISCUSSION
Decisive parameters for patient satisfaction following implantation of an AMS 800 are revision-free implant survival and satisfactory continence. While revision rates in the literature range from 4.5% to 59%, depending on the length of follow-up (12 months to 15 years) [2,4-8], our cohort had a revision rate of 29.1% with a median follow-up of 18 months. One reason for the comparatively high revision rate may lie in the fact that 10.5% of the patients were lost to follow-up. The most common reason for explantation was urethral erosion in 12.8% of cases, which is within the range Van der Aa et al. [2] describe in their review, though with a much longer follow-up (1.9%–28.6%). Van der Aa et al. [2] see most erosions in early years after implantation; another study group also describes a high incidence of erosion during the first 2 years after implantation [9]. In our cohort, both univariate and multivariate analysis revealed a significantly higher incidence of urethral erosion with small cuff sizes, particularly with 3.5- and 4-cm cuffs. This suggests a smaller urethral circumference due to preexistent urethral atrophy. Agarwal et al. [7] already described 3 causes of erosion in their publication: (1) intraoperative urethral injury, (2) patient factors promoting tissue atrophy like advanced age, and (3) irradiation as well as iatrogenic injuries. The latter are mainly responsible for late erosions and were the cause for revision in 1.4% of the cases in our cohort.
While in 1989 Scott still recommended a 4.5 cm cuff for all patients [10], later publications repeatedly called attention to the diversity of urethral structure. The anatomy of the urethra differs and tapers increasingly from the membranous to the penile segment [11,12]. This leads to a bias in our multicenter data; thus, implantation in a more proximal position could have led to larger cuff sizes and lower revision rate. Contrary to our results, Sandhu et al. [3] found that experienced surgeons tended to prefer smaller cuff sizes. A further potential bias of our study is that the cuff placement technique was not analyzed. A bulbospongiosus-sparing technique to reduce atrophy [6] was not performed in our participating centers. Transcorporal cuff placement was, however, applied in patients with small urethral circumferences, though precise analysis was not possible due to inconsistent/missing data. Other study groups also successfully applied the transcorporal technique for patients with small urethral circumferences [8,13]. In 2009 the 3.5-cm cuff was introduced for patients with a urethral circumference of less than 4 cm. Our cohort had the highest erosion rate with 3.5-cm cuffs (37.5%). In contrast, Simhan et al. [14] observed erosion in only 13% of their patients with 3.5-cm cuffs [5] and preferred them to transcorporal implantation or DCs. In other studies, 3.5-cm cuffs were not associated with a higher erosion risk, and the proportion of patients with 4-cm cuffs and persistent urethral incontinence was reduced by better cuff sizing. If erosion did occur, it was mostly in cases with prior irradiation [15,16]. Prior irradiation correlated significantly with urethral erosion in our analysis as well. This appears to be due to structural disruption with atrophy of the spongy tissue [14].
Rothschild et al. [17] called attention to the difference between urethral circumference and cuff size and was able to show that selecting the larger of 2 possible cuff sizes for a measured urethral circumference did not increase the incontinence rate. The high erosion rates suggest that the larger of 2 possible cuff sizes should be chosen for small urethras.
Multivariate analysis showed that the penoscrotal approach was independently associated with 2.1 times higher erosion rate than bulbar implantation. The results are in line with those of other study groups. While the erosion rate of 8% after 12 months first published by Wilson et al. [18] was tolerable, as in our cohort, later study groups recommend it only for revision surgery because of the significantly higher erosion and explantation rates [5,11]. When applying the penoscrotal approach, the cuff should be placed as far proximal as possible, as described by Wilson et al. [19] for the one-incision technique.
In our cohort, the double cuff AUS did not increase the erosion or general revision risk. However, Ahyai et al. [20] found a 5.7 times higher revision rate for bulbar double cuff implantation. In keeping with our results, another study group found no significant difference between SC and DC survival, but they noted a drifting apart of survival curves after 25 months [21]. As for cuff sizes, those applied had no significant influence on outcome with the DC, which was probably due at least partly to the use of 4.0 to 5.5 cm cuffs in 98.6% of the cases.
An established outcome measure is the achievement of social continence (0–1 pad/day). According to our observations, social continence was mostly achieved with a 4- or 4.5-cm SC or DC, though significance was not reached. Poorer continence rates were attributable to the high erosion rate with 3.5-cm cuffs and the high rate of persistent incontinence with cuff sizes ≥5 cm. Apart from persistent incontinence immediately after surgery, hypoxia-induced urethral atrophy can lead to urethral stricture and delayed recurrence of incontinence years later [8,16]. Due to its retrospective design and relatively short follow-up, our study could not conclusively evaluate atrophy-related late recurrence of incontinence.
Postoperative continence results could only be evaluated in 71% of our cohort. Analysis did not include preoperative data on continence for lack of comparable parameters. Favourable pad test and standardized questionnaires were used in a small number of patients only. This problem has already been critically discussed in a review on AUS by Van der Aa et al. [2]; most earlier publications do not report standardized data comparable to those of other study groups. The percentage of dry/socially continent patients is markedly lower in our cohort than hitherto published. This may be partly due to inclusion of patients from centers with a low level of expertise; the long learning curve has already been discussed [3]. Due to retrospective design of our multicenter DOMINO Project, precise information about surgeons’ experiences at the time of surgery was not available. The infection rate was low in our population and did not correlate significantly with cuff circumference. Undetected synchronous erosion is being discussed as the trigger; this view is supported by the temporal connection with frequent occurrence of both entities (i.e., erosion and infection) within the first 2 years [2,8].
Mechanical complications are only seen years after implantation [8] and were rare in our cohort.
Our study shows that, in a real-life setting in centers with varying expertise, implantation of the AMS 800 is not a simple intervention with consistently good continence results and low complication rates. Choosing the appropriate cuff is a difficult task, and the surgical procedure should be carefully considered, particularly for extreme urethral sizes. Urethral circumferences of 4.5 to 5.5 cm are associated with the greatest patient satisfaction. Our data suggest that patients with urethral atrophy and/or prior irradiation should perhaps receive a DC with the next larger cuff circumference and/or a transcorporal procedure for protection against erosion. Correct positioning of the measuring tape in the dorsal periurethral tissue should be doublechecked in patients with urethral circumferences ≥6 cm. Use of a DC was not associated with an increased erosion rate and led to improved continence outcomes. The higher erosion rate of the penoscrotal approach confirms the view taken by earlier study groups that this procedure should be restricted to revision surgery.
Notes
Research Ethics
This study was approved by the Institutional review Board (IRB) (Landesärztekammer Rheinland-Pfalz; Deutschhausplatz 3; 55116 Mainz; Germany; IRB number: 442/13). The written informed consent for publication could not have been obtained from all patients. Due to the fact that the research has been conducted in retrospect with long follow-up, it is not possible to inform every 473 patients. This practice was confirmed by IRB.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTION STATEMENT
· Full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis: FQ, TH, AK, RA, MK; RKH, TP, RO, AF, JP, CW, CMN, HL, JND, TH, EH, AO, AR, RH, RA, HL, RMB, AH, AJS
· Study concept and design: FQ, TH, AK, RA, HL, RMB, AH
· Acquisition of data: FQ, TH, AK, RA, MK; RKH, TP, RO, AF, JP, CW, CMN, HL, JND, TH, EH, AO, AR, RH, RA, HL, RMB, AH
· Analysis and interpretation of data: FQ, TH, AK, RMB, AH, AJS
· Drafting of the manuscript: FQ
· Critical revision of the manuscript for important intellectual content: FQ, TH, AK, RA, RMB, AH, AJS
· Statistical analysis: FQ, TH, AK, AJS
· Administrative, technical, or material support: FQ, TH, AK, RMB, AH, AJS
· Study supervision: FQ, TH, AK, RMB, AH, AJS
Acknowledgements
The author thanks all contributors involved in DOMINO Project especially those are not listed as coauthors for their help in data-collection and editing the manuscript.