Neuregulin-1 Protects Neuronal Cells Against Damage due to CoCl2-Induced Hypoxia by Suppressing Hypoxia-Inducible Factor-1α and P53 in SH-SY5Y Cells
Article information
Abstract
Purpose
Hypoxia-mediated neurotoxicity contributes to various neurodegenerative disorders, including Alzheimer disease. Neuregulin-1 (NRG1) plays an important role in the development and plasticity of the brain. The aim of the present study was to investigate the neuroprotective effect and the regulating hypoxic inducible factor of NRG1 in cobalt chloride (CoCl2) induced hypoxia.
Methods
Hypoxia was induced in SH-SY5Y cells by CoCl2 treatment. SH-SY5Y cells were pretreated with NRG1 and then treated with CoCl2. Western blotting, immunocytochemistry, and lactate dehydrogenase (LDH) release assays were performed to examine neuroprotective properties of NRG1 in SH-SY5Y cells.
Results
Our data showed that CoCl2 induced cytotoxicity and changes of hypoxia-inducible factor-1α (HIF-1α) and p53 expression in SH-SY5Y cells. However, pretreatment with NRG1 inhibited CoCl2-induced accumulation of HIF-1α and p53 stability. In addition, NRG1 significantly attenuated cell death of SH-SY5Y induced by CoCl2.
Conclusions
NRG1 can regulate HIF-1α and p53 to protect neurons against hypoxic damage.
• HIGHLIGHTS
- CoCl2 induced cytotoxicity and changes of HIF-1α and p53 expression in SH-SY5Y cells.
- NRG1 inhibited CoCl2-induced accumulation of HIF-1α and p53 stability.
- NRG1 attenuated SH-SY5Y cell death induced by CoCl2.
INTRODUCTION
Brain requires a continuous supply of the largest amount of oxygen to maintain its normal function. Thus, brain is especially sensitive to hypoxia, a situation where the amount of oxygen is limited. Hypoxia can cause abnormal structural and functional alterations, eventually leading to cell death [1]. Several studies have shown that damage to the brain due to loss of oxygen is closely related to neurodegenerative diseases such as vascular dementia, Alzheimer disease, and Parkinson disease [2,3]. In hypoxic brain, accumulation of hypoxia-inducible factor-1α (HIF-1α) can be observed in the several brain regions including the cerebral cortex and the hippocampus [4,5]. HIF-1α is rapidly degraded by the ubiquitin-proteasome pathway [6,7] and kept at very low levels under normoxic conditions. However, it is stabilized due to attenuation of prolyl hydroxylase [8] caused by hypoxia. It is translocated into the nucleus and forms a heterodimeric complex with HIF-1β.
Upon hypoxia, HIF-1α is activated, inducing several genes involved in multiple processes including glucose uptake, glycolysis, angiogenesis, cell survival, and cell invasion [9,10]. The ability of p53 to suppress tumorigenesis is fundamentally linked to its central role in the cellular response to stress. Although normally low or undetectable, p53 level and activity are rapidly increased in response to a wide range of stresses, including hypoxia, DNA damage, oncogene activation, microtubule disruption, and oxidative damage [11,12]. P53-mediated neuronal stress is implicated in neurological damage that occurs following acute insults such as ischemia, traumatic brain injury, and exposure to neurotoxins. Sustained p53-mediated apoptosis is also associated with chronic neuron degenerative pathologies, including Alzheimer’s disease and Huntington disease [13,14].
Neuregulin-1 (NRG1) is a member of a family of proteins containing an epidermal growth factor-like motif that mediates important functions in the nervous system [15,16]. It has been shown that NRG1 can regulate nerve cell differentiation, neuron migration, neurotransmission, neurite outgrowth, and synaptic activity [16,17]. NRG1 can protect neurons under various pathological conditions, including ischemia and oxidative stress [18,19]. We have previously reported neuroprotective effects of NRG1 signaling in cell models and Tg2576 mice models [20,21]. Furthermore, we have found that NRG1 is significantly reduced in human AD brains and that it regulates non-amyloidogenic processing in primary cortical neurons. However, no study has reported the role of NRG1 in the expression of hypoxic inducible protein in neuronal cells. Thus, the objective of the present study was to evaluate effects of NRG1 on cobalt chloride (CoCl2)-induced hypoxic stress in SH-SY5Y cells. In addition, effects of NRG1 on hypoxic inducible factors such as HIF-1α and p53 were characterized.
MATERIALS AND METHODS
Materials and Antibodies
Recombinant human NRG1 was purchased from PROSPEC (East Brunswick, NJ, USA). Cobalt chloride (CoCl2, C8661) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Antibodies for HIF-1α and NB100-131 were supplied by Novus Biologicals (Centennial, CO, USA). Antibodies for p53 (sc-126), β-actin (sc-47778), HRP-conjugated anti-rabbit IgG (sc-2004), and HRP-conjugated anti-mouse IgG (sc-2005) were obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA).
Cell Culture
SH-SY5Y human neuroblastoma cells were provided from the American Type Culture Collection (Manassas, VA, USA). These cells were cultivated in Dulbecco’s Modified Eagle’s Medium (Invitrogen, Carlsbad, CA, USA) supplement with a penicillin-streptomycin-amphotericin B solution (Invitrogen) and 10% fetal bovine serum at 37°C with 5% CO2.
Lactate Dehydrogenase Release Assay
Cytotox 96 nonradioactive cytotoxicity assay kit (Promega, Madison, WI, USA) was employed according to the instructions of the manufacturer. Accordingly, 10 μL of the cell supernatants were mixed with 100-μL lactate dehydrogenase (LDH) reaction reagent at room temperature for 30 minutes. Absorbance was determined by using an enzyme-linked immunosorbent assay reader (Victor X3, PerkinElmer, Shelton, CT, USA) with filter for 490 nm.
Immunocytochemistry
SH-SY5Y cells were carried out as previously described [22]. SH-SY5Y cells were fixed with 4% paraformaldehyde in phosphatebuffered saline (PBS) (pH, 7.4) at room temperature for 20 minutes. The cells were then washed with PBS 3 times and permeabilized with 0.1% Triton X-100 for 30 minutes. After incubation, cells were blocked with 10% normal horse serum for 30 minutes. The cells were incubated with indirect immunofluorescence using a primary antibody to p53 (1:100) and HIF-1α (1:100) at 4°C overnight. The cells were labeled by fluorescein isothiocyanateconjugated secondary antibody (1:200; Jackson Immuno Research Laboratories, Inc.) for 2 hours at room temperature. Nuclei were counterstained with 4′-6-diamidino-2-phenylindole (Invitrogen, 10μM) for 30 minutes. Imunostained cells were captured in at least 5 random fields using a Zeiss LSM 5 LIVE confocal microscope (Carl Zeiss AG, Oberkochen, Germany).
Western Blot Analysis
Protein samples (30–50 μg) from SH-SY5Y cells lysed in radio immunoprecipitation assay buffer was loaded onto sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a NC membrane (Millipore, Burlington, MA, USA). Membranes were incubated with the appropriate primary antibodies (anti-HIF-1α, 1:1,000, Novus Biologicals; anti-p53, 1:1,000, Santa Cruz Biotechnology; anti-β-actin, 1:5,000, Santa Cruz Biotechnology). After washing with TBS-T (Tris-buffered saline, 0.1% Tween 20) 3 times at RT, membranes were further incubated with horseradish peroxidase-conjugated goat secondary antibodies. The immunoreactive bands were shown using an enhanced chemiluminescent substrate (Bio-Rad Laboratories, Richmond, CA, USA).
Data Analysis
All results are presented as mean±standard error of the mean. Student t-test and 1-way analysis of variance with Bonferroni post hoc test were used to analyze statistical significance (GraphPad Prism 6, CA, USA). P<0.05 was assumed for statistical significance.
RESULTS
CoCl2 Induces Cytotoxicity and Change of HIF-1α and p53 Protein Expression in SH-SY5Y Cells
CoCl2 has been widely used to induce hypoxia mimetic condition both in vitro and in vivo. First, we examined effects of CoCl2-induced cytotoxicity in SH-SY5Y cells. LDH assays showed that CoCl2 treatment significantly increased cell death in a time-dependent manner. In particular, cell death was significantly increased after exposure to 100μM CoCl2 for>12 hours compared with that of the control group. However, LDH release continued to increase in each subsequent time interval (0, 3, 12, 24, 36, 48 hours) (Fig. 1A). As shown in Fig. 1B and C, CoCl2 induced HIF-1α protein expression in SH-SY5Y cells at 6 hours after treatment. HIF-1α protein expression peaked at 12 hours. This peak sustained through 48 hours after CoCl2 treatment. Moreover, the expression of p53 was markedly enhanced in CoCl2-induced SH-SY5Y cells in a time-dependent manner.
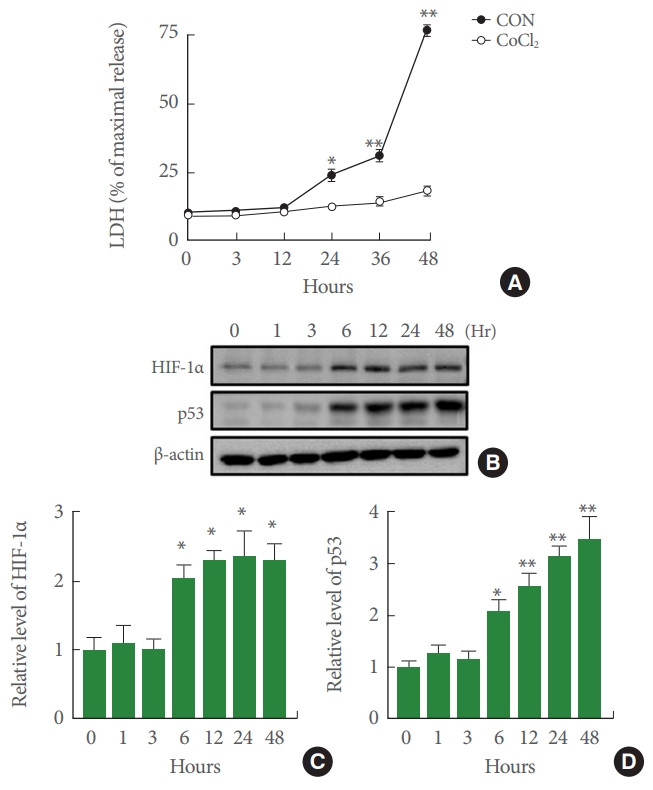
Effects of cobalt chloride (CoCl2) on cytotoxicity and protein levels of hypoxia-inducible factor-1α (HIF-1α) and p53 in SH-SY5Y cells. (A) Cell death was detected by lactate dehydrogenase (LDH) release assay following treatment with CoCl2 or serum-free medium for indicated time periods. Effects of CoCl2 on LDH release in cultured cells for 3, 12, 24, 36, and 48 hours of 100μM CoCl2 treatment were determined (n =4, *P<0.05, **P<0.01). (B) Western blotting demonstrated that 100μM CoCl2 affected HIF-1α and p53 protein expression levels. Both HIF-1α and p53 protein expression were significantly increased by 100µM CoCl2 treatment in a time dependent manner. (C, D) Quantification of data in B. Densitometry values are shown as ratios relative to values of the control group (n=6, *P<0.05, **P<0.01).
NRG1 Pretreatment Reduces CoCl2-Induced Accumulation of HIF-1α in SH-SY5Y Cells
The key regulator of hypoxia response is HIF-1. HIF-1 is a complex heterodimer including a variety of subunits (HIF-1α and HIF-1β), of which HIF-1α causes hypoxia. To investigate whether NRG1 affected CoCl2-induced increase in HIF1-1α expression, cells were pretreated with 5nM NRG1 followed by 24 hours of CoCl2 treatment. Western blotting was then used to examine levels of HIF-1α in SH-SY5Y cells. Results confirmed that treatment of SH-SY5Y cells with both low (100μM) and high (500μM) concentrations of CoCl2 for 24 hours significantly upregulated levels of HIF-1α (control [CON], 1.00±0.17; 100μM CoCl2, 2.67±0.26; n=6, *P<0.05; CON, 1.13±0.09; 500μM CoCl2, 3.37±0.21, n=6; **P<0.01). Moreover, pretreatment with 5nM NRG1 for 24 hours attenuated such increase in HIF-1α expression induced by treatment with CoCl2 (100μM CoCl2, 2.67±0.26; 100μM CoCl2+NRG1, 1.17±0.19, 500μM CoCl2, 3.37±0.21, 500μM CoCl2+NRG1, 1.60±0.31, n=6; ##P<0.01) (Fig. 2A, B). The immunoreactivity of HIF-1α in SH-SY5Y cells was measured by immunofluorescence.
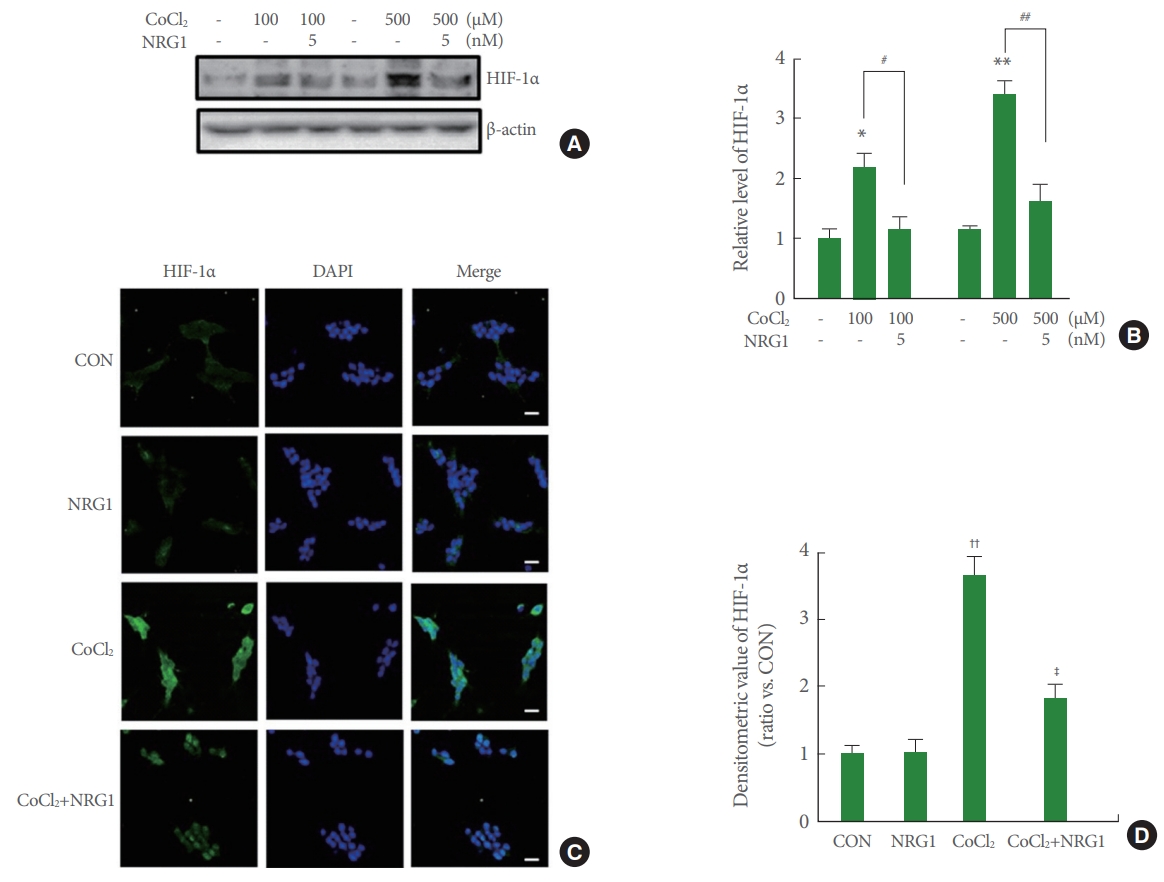
Neuregulin-1 (NRG1) attenuates cobalt chloride (CoCl2)-induced accumulation of hypoxia-inducible factor-1α (HIF-1α) in SH-SY5Y cells. (A) HIF-1α accumulated upon treatment with 100μM CoCl2 or 500μM CoCl2 and CoCl2 -induced HIF-1α accumulation was inhibited by 5nM NRG1 treatment (15 minutes of NRG1 pretreatment followed by 6 hours of CoCl2 treatment). (B) Quantification of data in B. Densitometry values are shown as ratios relative to values of the control (CON) group (n=6, *P<0.05, **P<0.01, #P<0.05, ##P<0.01). (C) Expression of HIF-1α after 24 hours of incubation under control, 10nM NRG1, 100μM CoCl2, or treatment with 10nM NRG1 in SH-SY5Y cells by immunofluorescence. SH-SY5Y cells were fixed and immunostained with anti-HIF-1α (green), while 4′-6-diamidino-2-phenylindole (DAPI) (blue) was used as a counterstain. Scale bars represent 20 μm. (D) Fluorescence density of HIF-1α was measured in each group (n=8, ††P<0.01, ‡P<0.05, †compared to the CON group; ‡compared to the CoCl2 group).
To examine effects of NRG1 in SH-SY5Y, cells were pretreated with 10nM NRG1 and then treated with 100μM CoCl2 15 minutes later. Treatment with 100μM CoCl2 for 24 hours significantly upregulated HIF-1α expression and increased nuclear localization in comparison with that of the control (CON, 1.00±0.12; CoCl2, 3.65±0.28, n=8; ††P<0.01). Treatment with 10nM NRG1 for 24 hours attenuated the increase in HIF-1α immunoreactivity induced by treatment with CoCl2 (CoCl2, 3.65±0.28; CoCl2+NRG1, 1.83±0.20, n=8; ‡P<0.05) (Fig. 2C, D). These results of immunoreactivity were consistent with western blot assay results.
NRG1 Decreases p53 Stability Induced by CoCl2 in SH-SY5Y Cells
Hypoxic stress could induce stabilization of p53 implicated in regulation of cell fate. To explore the role p53 in response to CoCl2 stress, the expression of p53 protein in SH-SY5Y cells was examined. Results showed a dose-dependent increase inp53 expression by 24 hours of treatment with CoCl2 (50–200μM) (Fig. 3A). Quantification demonstrated that CoCl2 significantly increased the expression of HIF-1α (CON, 1.00±0.20; 50μM CoCl2, 1.36±0.15; 100μM CoCl2, 2.40±0.146; 150μM CoCl2, 3.30±0.30; 200μM CoCl2, 3.67±0.42, n=6) (Fig. 3B). Effect of NRG1 in SHSY5Y cells under hypoxic condition was also investigated. As shown in Fig. 3C, treatment with CoCl2 significantly increased p53 stabilization for the indicated time (24 hours: CON, 1.00±0.12; CoCl2, 2.80±0.15; 48 hours: CON, 1.60±0.21; CoCl2, 3.23±0.20, n=6) (Fig. 3C, D). Treatment with 10nM NRG1 for 24 or 48 hours attenuated the increase in p53 stabilization induced by treatment with 100μM CoCl2 (24 hours: CoCl2, 2.80±0.15; CoCl2+NRG1, 1.73±0.24; 48 hours: CoCl2, 3.23±0.20; CoCl2+NRG1, 1.70±0.25, n=8; #P<0.05) (Fig. 3C, D).

Neuregulin-1 (NRG1) inhibits cobalt chloride (CoCl2)-induced p53 stability in SH-SY5Y cells. (A) SH-SY5Y cells were treated with various concentrations (0, 50, 100, 150, and 200µM) of CoCl2 for 24 hours, which resulted in dose-dependent increase of p53 stability. (B) Quantification of data in A. Densitometry values are shown as ratios relative to values of the control (CON) group (n=6, *P<0.05 vs. control group, #P<0.01 vs. control group). (C) NRG1 decreased CoCl2-induced p53 stability. SH-SY5Y cells were incubated with or without 100μM CoCl2 for indicated times, or with pretreatment of NRG1 (5 or 10nM) 15 minutes prior to CoCl2 treatment. (D) Quantification of data in C. Densitometry values are shown as ratios relative to values of the CON group (n=6, *P<0.05 vs. control group, #P<0.05 vs. CoCl2-treated group).
NRG1 Inhibits CoCl2-Induced Expression and Nuclear Accumulation of p53 in SH-SY5Y Cells
Immunocytochemistry was performed to investigate expression and localization of p53 in SH-SY5Y cells. To investigate effects of NRG1, SH-SY5Y cells were pretreated with 10nM NRG1 and then treated with 100μM CoCl2 15 minutes later. Treatment with CoCl2 for 24 hours resulted in markedly increased unclear localization of p53 and upregulated p53 expression in comparison with the control (CON, 1.00±0.17; CoCl2, 3.85±0.54, n=8; **P<0.01). Moreover, treatment with 10nM NRG1 inhibited the increase in p53 immunoreactivity induced by treatment with CoCl2 (CoCl2, 3.85±0.54; CoCl2+NRG1, 1.81±0.20, n=8; #P<0.05) (Fig. 4A, B).
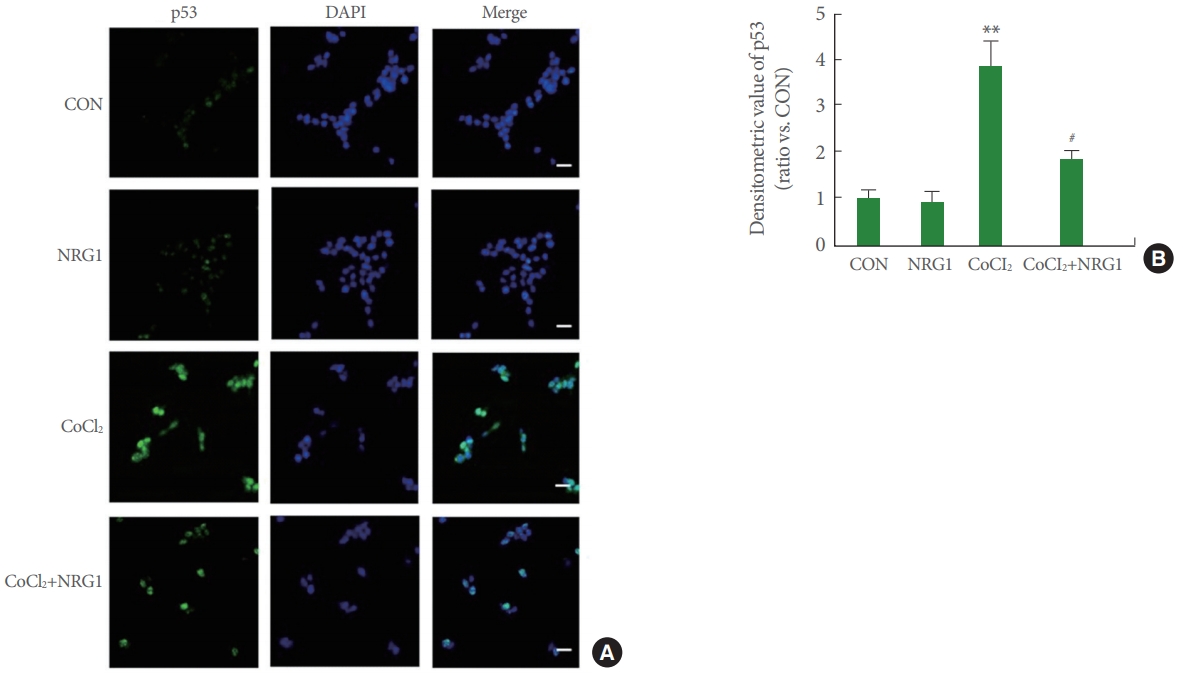
Neuregulin-1 (NRG1) attenuates the expression and nuclear accumulation of p53 induced by cobalt chloride (CoCl2) in SH-SY5Y cells. (A) Expression of p53 after 24 hours incubation under control, 10nM NRG1, 100μM CoCl2, or treatment with 10nM NRG1 in SH-SY5Y cells by immunofluorescence. Cells were double-labeled with 4′-6-diamidino-2-phenylindole (DAPI) (blue) and anti-p53 (green) and then analyzed by fluorescence microscopy. Scale bars represent 20 μm. (B) Fluorescence density of p53 was measured in each group (n =8, **P<0.01, #P<0.05, *compared to the control [CON] group; #compared to the CoCl2 group).
NRG1 Prevents SH-SY5Y Cell Death Induced by CoCl2
To determine whether NRG1 could affect the observed cytotoxicity in SH-SY5Y cells induced by CoCl2 treatment, an LDH release assay was performed following 24 hours of CoCl2 treatment. Cells were incubated with different concentrations (5 and 10nM) of NRG1 and then exposed to 100μM CoCl2 for 24 hours. NRG1 at both concentrations (5 and 10nM) attenuated neuronal cell death induced by 100μM CoCl2 treatment in SH-SY5Y cells (CON, 10.61±1.73; 5nM NRG1, 9.55±1.14; 10nM NRG1, 9.89±1.52; CoCl2, 30.49±1.25; CoCl2+NRG1 5nM, 19.20±2.63; CoCl2+10nM NRG1, 12.98±1.96, n=6; **P<0.01, #P<0.05, ##P<0.01) (Fig. 5A). Representative images of cells are shown in Fig. 5B. These results indicate that NRG1 exerts a significant protective effect over cell death induced by CoCl2 treatment.
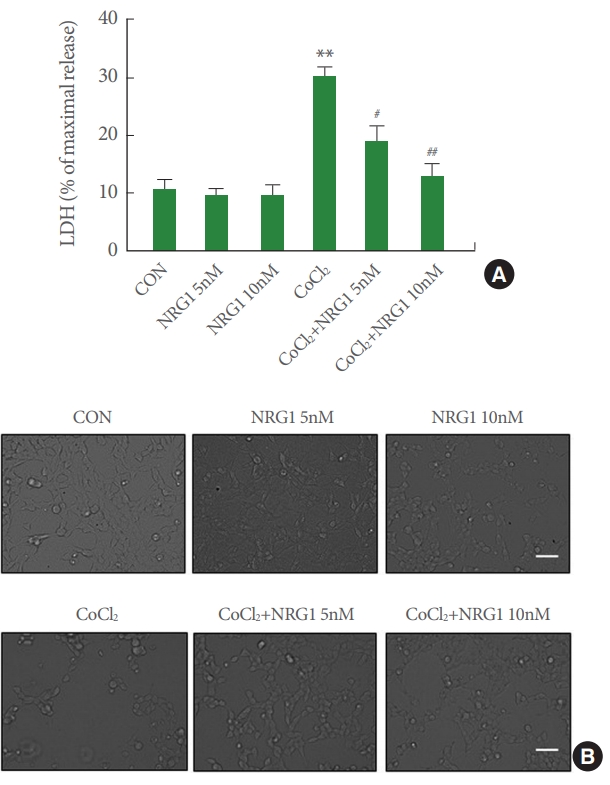
Neuregulin-1 (NRG1) attenuates SH-SY5Y cell death induced by cobalt chloride (CoCl2). (A) NRG1 (5 or 10nM) attenuated the cell death induced by 100μM CoCl2 in SH-SY5Y cells. After 24 hours, the degree of cell death was assessed by LDH activity in the medium (n =6, **P <0.01 vs. control group, #P<0.05 vs. CoCl2 treated group, ##P<0.01 vs. CoCl2 treated group). (B) Representative photographs of cells. Scale bar represents 100 μm. CON, control; LDH, lactate dehydrogenase.
DISCUSSION
In this study, we determined whether NRG1 was implicated in hypoxic stress induced by CoCl2 using in vitro cell models. CoCl2 has been used as a hypoxia-mimicking agent in both in vivo and in vitro studies to generate hypoxia like environment [23,24] by inducing the accumulation of HIF-1α [25]. It can also up-regulate genes such as p53, p21, and PCNA [26]. Our present study confirmed that CoCl2 treatment increased the cytotoxicity and protein levels of HIF-1α and p53 in SH-SY5Y cells in a time-dependent manner. These observations suggest that the model of hypoxic stress induced by CoCl2 is a suitable tool to investigate mechanisms of neuronal cell injury.
NRG1 is expressed in multiple regions of the adult nervous system on top of the developing brain. NRG1 is a growth factor those signaling plays important roles in the maintenance of brain circuits [27]. Several lines of evidence collectively suggest that NRG1 plays a protective role in neurons against neurotoxic stimuli including ischemic insult, MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine), and H2O2 [18,28]. In a previous study, we have found that NRG1/ErbB4 signaling exerts neuroprotective effects against the Swedish mutant amyloid precursor protein, amyloid beta-peptide Aβ1-42, and C-terminal fragments of amyloid precursor protein [19,21]. We have also shown that NRG1 can prevent Aβ1-42-induced impairment of LTP by its ErbB4 receptor [29]. In addition, we have recently reported that NRG1 can attenuate H2O2-indcuced oxidative stress [30]. NRG1 is also found to play a key role in the peripheral nerves and skeletal muscle [31]. Glial growth factor 2 (neuregulin-1β3 type II) promotes the recovery of erectile function in a rat model of radical prostatectomy-associated cavernous nerve injury [32].
To investigate whether NRG1 might affect the increase of HIF-1α expression induced by CoCl2, we evaluated HIF-1α levels via western blotting and immunocytochemistry. We observed that NRG1 attenuated CoCl2-induced accumulation of HIF-1α in SH-SY5Y cells. Especially, NRG1 significantly inhibited the increase of nuclear localization of HIF-1α. These results provide evidence that NRG1 signaling could directly regulate hypoxic dysfunction of neurons.
Under hypoxic conditions, HIF-1α accumulates and triggers the activation of many genes. It has been reported that HIF-1α can cause hypoxia-induced apoptosis by mediating stabilization of p53 and its transactivation under hypoxic conditions. The p53 is a potent transcription factor that activates target genes to initiate apoptosis [33]. In normal cells, p53 is maintained at low levels by ubiquitin ligase mouse double minute 2 homolog (MDM2)-mediated degradation. It is strongly stabilized in response to various types of stress, including hypoxia [34]. HIF-1α can increase the stability of wild-type p53 by directly binding to the p53 ubiquitin ligase MDM2 [33].
Upon exposure to a variety of stress conditions, the stability of p53 increases and the protein accumulates in the nucleus, where it is activated as a transcription factor [35]. To explore the implication of p53 in response to CoCl2 stress, we monitored CoCl2-induced p53 stability. We found that p53 was upregulated by CoCl2 treatment dose-dependently. Moreover, NRG1 ameliorated CoCl2-induced p53 stability in SH-SY5Y. Furthermore, NRG1 markedly decrease unclear localization of p53 and upregulated p53 expression. Taken together, our results suggest that NRG1 can regulate HIF-1α and p53 to protect neurons from hypoxic damage. Further research is needed to clarify the regulation effect of NRG1 on mechanism of under signaling.
Notes
Fund/Grant Support
This work was partly supported by grants (NRF-2016R1A2B4010574 awarded to RSW and 2017R1D1A1B03028729 awarded to JHL) of the National Research Foundation of Korea funded by the Ministry of Education, Science, and Technology.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTION STATEMENT
·Full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis: JHL, RSW
·Study concept and design: JHL, RSW
·Acquisition of data: SYY, JYY, HBK
·Analysis and interpretation of data: RSW
·Drafting of the manuscript: SYY, RSW
·Critical revision of the manuscript for important intellectual content: RSW
·Statistical analysis: TKB
·Obtained funding: JHL, RSW
·Administrative, technical, or material support: JYY
·Study supervision: RSW
