Robotic Sacrocolpopexy for Treatment of Apical Compartment Prolapse
Article information
Abstract
Abdominal sacrocolpopexy is the gold-standard treatment for apical compartment prolapse, as it is more effective and durable than the transvaginal approach. In the current era of minimally invasive surgery, laparoscopic sacrocolpopexy techniques have been described, but have not gained popularity due to their complexity and steep learning curves. To overcome this problem, robotic sacrocolpopexy was introduced, and has shown equivalent outcomes and safety compared to open and laparoscopic sacrocolpopexy based on findings that have been accumulated over 15 years.
INTRODUCTION
The incidence of pelvic organ prolapse (POP) has been gradually increasing with the global increase in the aging population, and the lifetime risk of symptomatic vaginal prolapse requiring surgical treatment is estimated to be 12.6% [1,2]. Although anterior vaginal prolapse is the most common type of prolapse, apical prolapse is also highly prevalent. It has been estimated that 1 in 9 women undergoes hysterectomy during her lifetime, and surgical treatment is required in 10% of them due to symptomatic vaginal prolapse [3]. Moreover, apical support defects after hysterectomy are a risk factor for POP and increase the possibility of vault prolapse.
abdominal sacrocolpopexy (ASC) remains the gold-standard procedure for apical compartment prolapse, as it offers superior outcomes for a variety of vaginal procedures with few complications [3]. In addition to its high success rate and durable results, sacrocolpopexy can maintain the normal axis of the vagina and maximal vaginal length by fixing the vaginal apex on the anterior surface of the sacrum. Sacrocolpopexy is traditionally performed via laparotomy; however, minimally invasive approaches have been developed to overcome concerns about the increased risk of morbidity associated with open surgery, in addition to long surgery times and lengthy hospital stays. While reports have indicated that laparoscopic sacrocolpopexy (LSC) has the advantages of a shorter hospital stay and less blood loss, surgical time is not significantly shorter, and this technique has a longer learning curve than traditional sacrocolpopexy [4].
To address these issues, robotic sacrocolpopexy (RSC) has been explored. After Di Marco et al. [5] published the first case series involving 5 patients who underwent RSC in 2004, many more reports have been published. The ultimate advantages of RSC are the 3-dimensional view afforded by the use of robotic instruments, the increased degree of freedom in movement, elaborate suturing ability, and easy knot-tying. These advantages of robotic surgery can overcome the technical limitations and steep learning curve associated with LSC. In this review, we discuss the efficacy and safety of RSC, as well as the latest trends in this field.
SURGICAL TECHNIQUE
RSC consists of 3 main steps: vaginal dissection, presacral peritoneal dissection, and mesh fixation. However, as shown in Table 1, the type of mesh, suture material, and depth of vaginal dissection vary among studies.
The shape of the mesh used (Y-shaped mesh, 2 separate meshes, T-shaped mesh, or racket-shaped mesh) varies depending on the surgeon, and the depth dissected is often not mentioned (Table 1). Thus far, there are no clear rules as to the extent of anterior dissection that should be performed. A commonly accepted rule is to dissect as distally as possible to prevent recurrence, but not below the trigone. We apply this rule to dissect the anterior vaginal wall distally to just above the level of the trigone (3–5 cm distal to the vaginal apex), and the posterior wall to the midpoint (Fig. 1). We use prefashioned Y-shaped DynaMesh, which is a polyvinylidene fluoride monofilament material, for sacrocolpopexy or hysteropexy (for uterine preservation). In patients who have previously undergone hysterectomy, the anterior and posterior vaginal walls are dissected under guidance of a vaginal manipulator, and then the distal 2-arm of mesh is fixed to the anterior and posterior vaginal walls (Fig. 2). When the uterus has been preserved, the mesh is transferred through the broad ligament with a proximal arm on the right side (Fig. 3).
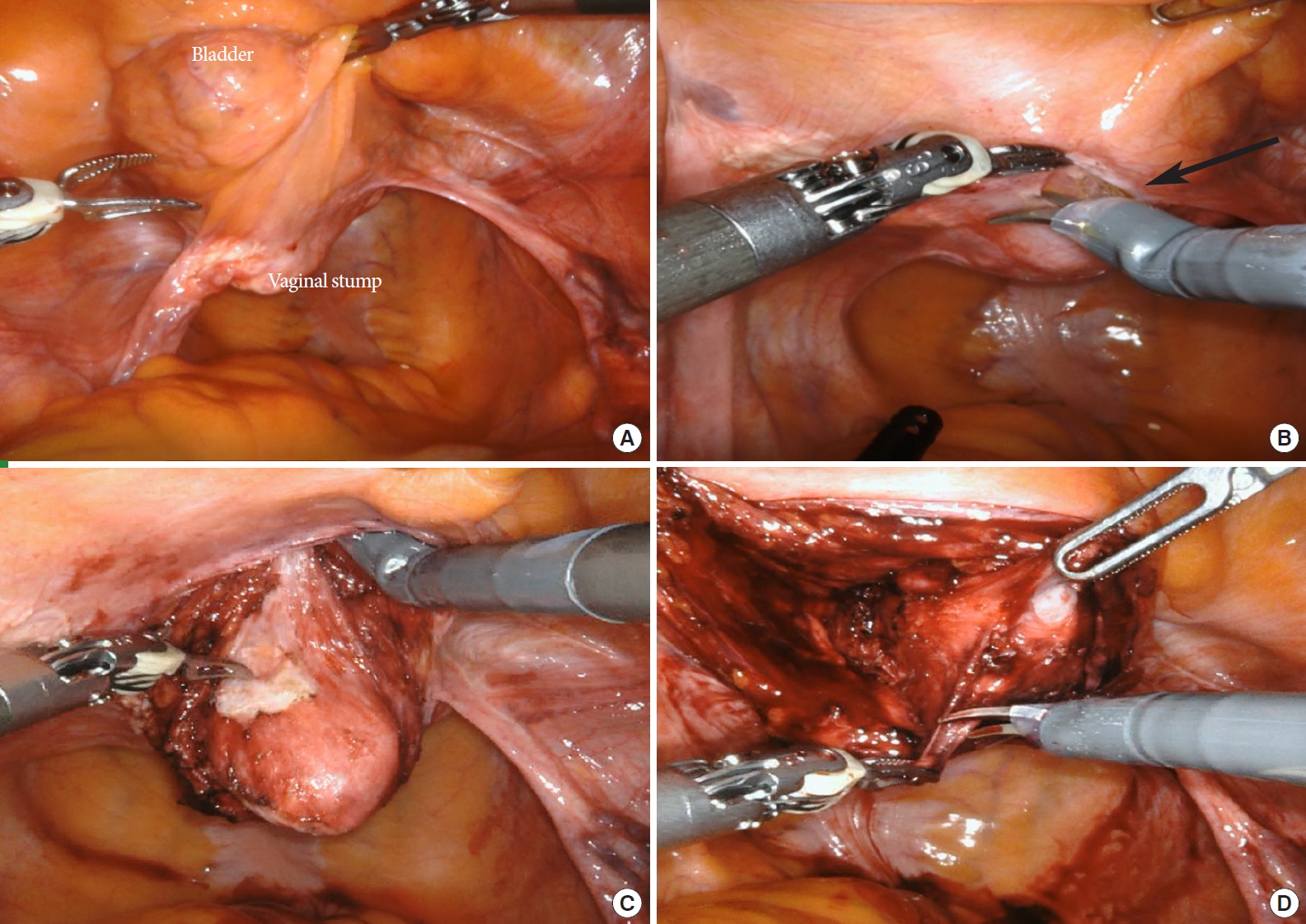
Vaginal wall dissection (posthysterectomy vaginal vault prolapse). (A) Surgical anatomy of the vaginal apex and bladder, (B, C) anterior vaginal wall dissection (black arrow: vesicovaginal junction), and (D) posterior vaginal wall dissection.
Fixation of the distal mesh arms to (A, B) the anterior vaginal wall and (C) the posterior vaginal wall using a barbed, delayed-absorbable suture (V-Loc 180; Covidien, Walpole, MA, USA) in a continuous manner. (D) The proximal arm of the mesh passes through the posterior peritoneum (yellow arrow).

Vaginal wall dissection in sacrohysteropexy (uterus-preserving). (A) Surgical anatomy of the vaginal apex and bladder. (B) Anterior vaginal wall dissection. (C) Posterior vaginal wall dissection. (D) The anterior mesh arm is tunneled through the right broad ligament. Fixation of the distal mesh arms to (E) the posterior vaginal wall and (F) the anterior vaginal wall using a barbed, delayed-absorbable suture (V-Loc 180; Covidien, Walpole, MA, USA) in a continuous manner.
Currently, the consensus is to affix the sacral arm of the mesh to the most superior point of the anterior surface of S1. However, although the bleeding risk is lower at the S1 level than at other levels, the promontory is not anatomically familiar, and there is a risk of bleeding due to the close proximity of nerves to the surrounding blood vessels. Therefore, caution is required when performing dissection.
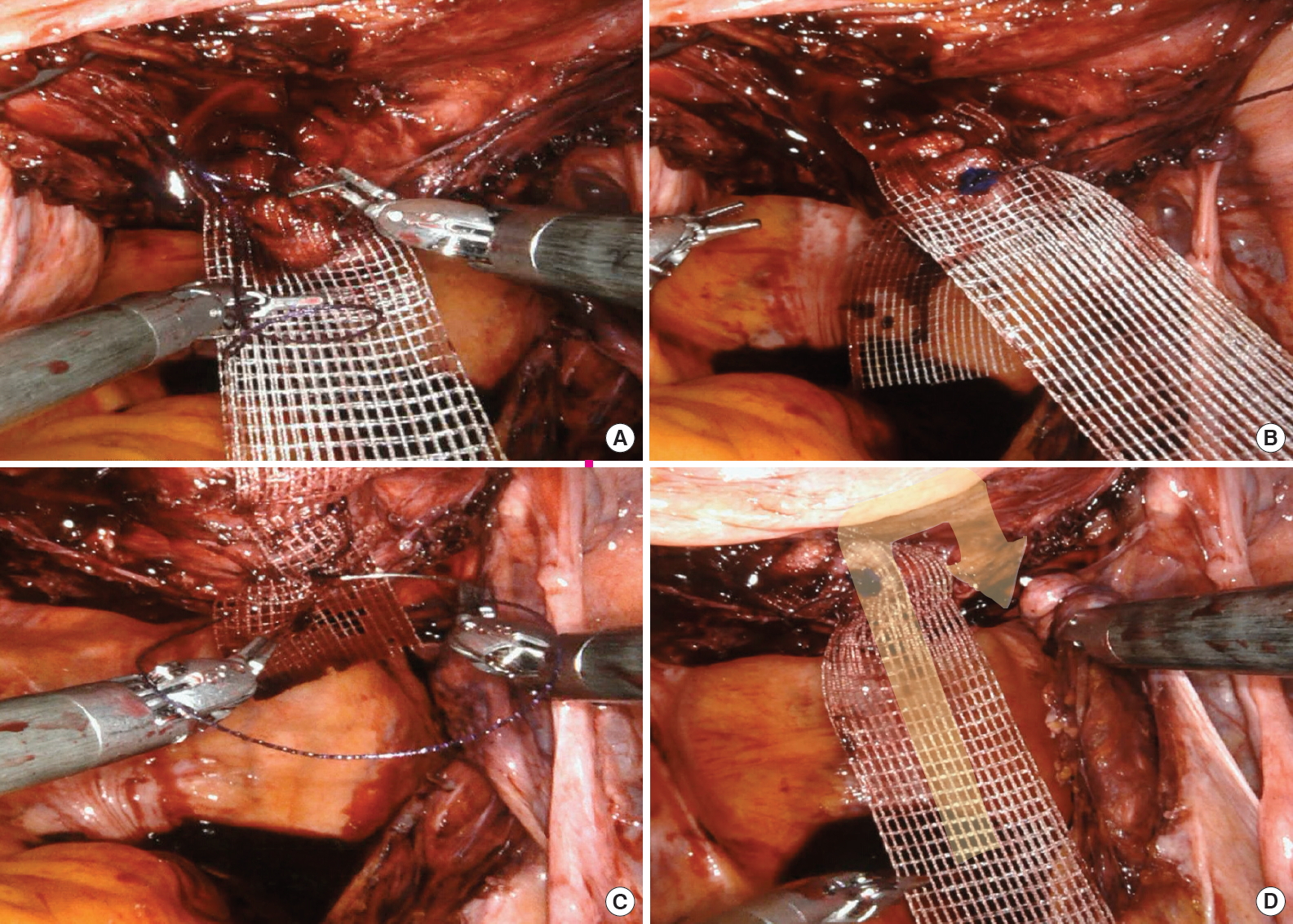
A nonabsorbable suture is used in traditional open sacrocolpopexy to prevent detachment of the mesh from the vagina and sacral promontory and to decrease the risk of mesh exposure and suture erosion. In a series of RSCs with a median of 33 months of follow-up, the use of absorbable sutures for both vaginal and sacral mesh attachment was effective; the 3-year rate of survival without repeat prolapse surgery was 93% [6]. Tan-Kim et al. [7] introduced a technique that fixes mesh to the vaginal wall with a barbed absorbable suture and showed that the nonbarbed suture group had significantly longer operative times than the barbed suture group (42 minutes vs. 29 minutes, P<0.001) without any significant difference in anatomic failure between groups at 12 months. Currently, we use a barbed, delayed-absorbable suture (V-Loc 180; Covidien, Walpole, MA, USA) to fix the mesh to the vaginal wall in a continuous manner (Figs. 2, 3). In the next step, with the vagina restored to its normal position, the proximal arm of the mesh is fixed to the promontory without excessive tension (Fig. 4). Finally, the peritoneum is repaired using a barbed, delayed-absorbable suture.

Fixation of the proximal arm of the mesh. (A) At this time, the tension of the mesh should be adjusted while the vagina is restored using a vaginal manipulator. (B) The mesh is sutured to the anterior longitudinal ligament overlying the sacrum with 2–3 sutures. (C) Reperitonealization after fixation of the mesh using a barbed, delayed-absorbable suture (V-Loc 180; Covidien, Walpole, MA, USA) in a continuous manner.
SURGICAL OUTCOMES
Outcomes of RSC and Heterogeneity of Surgical Methods
With regard to the efficacy of RSC, the objective cure rate was reported to be 84%–100% and the subjective cure rate to be 92%–95% in a systematic review that analyzed studies conducted between 2006 and 2013 [8]. Studies published since then have reported similar efficacies (Table 2). However, there are major challenges when comparing research results. First, the definitions of success and failure differ among studies. Second, follow-up periods vary substantially among studies. Third, preoperative POP severity also varies among patients. Finally, a considerable amount of heterogeneity is observed at each stage of the surgical technique. Therefore, interpretation of outcomes requires caution.
In studies with an average follow-up period of 6 months to 12 months, the cure rate of apical compartment prolapse ranges from 88%–100% [9-15], and in those with a follow-up period of 12–24 months, the cure rate ranges from 91.4%–100% [6,16-22]. Even in long-term studies with over 5 years of follow-up, the cure rate is 93.3%–100%, indicating that RSC has excellent durability [23,24] (Table 2).
In studies conducted only on patients with advanced-stage POP, namely Baden-Walker grade 3 or Pelvic Organ Prolapse Quantification System (POP-Q) stage 3 or higher, the anatomical cure rate of apical compartment prolapse is 95%–100%, indicating excellent outcomes [16-18,22,24-26].
Concurrent Supracervical Hysterectomy or Uterine Preservation
Because apical compartment prolapse includes not only vault prolapse that occurs in patients who have undergone prior hysterectomy, but also uterine prolapse, many studies are not limited to patients who have undergone hysterectomy. Therefore, when RSC is performed on patients with a uterus, concurrent hysterectomy or supracervical hysterectomy is performed depending on the surgeon, although hysteropexy can be performed with uterus preservation. In addition, supracervical hysterectomy and uterine preservation are performed selectively depending on the patient, even in the same study. However, in most studies, the surgical outcomes of concurrent supracervical hysterectomy or uterine preservation have not been analyzed separately. As shown in Table 1, the objective cure rate of apical compartment prolapse was reported to be 88%–100% in 5 studies where RSC was performed only on patients suffering from vault prolapse after hysterectomy [13,15,16,24]. Among 12 studies in which RSC was performed on patients suffering from apical compartment prolapse irrespective of prior hysterectomy (excluding 4 studies where it was unclear whether the uterus was preserved or removed during RSC), 2 studies where the uterus was always preserved reported a cure rate of 100% [17,27], and 5 studies of concurrent supracervical hysterectomy in all patients reported cure rates ranging from 93.3% to 99.3% [11,12,14,23,25]. All other studies preserved the uterus or performed concurrent supracervical hysterectomy depending on the patient, with reported cure rates ranging from 94% to 100% [9,10,18,20,21,28]. Van Zanten et al. [21] performed the first prospective study of patients with symptomatic apical POP of POP-Q stage 2 or higher. In that study, the authors compared 188 patients who had undergone RSC with prior hysterectomy and 117 patients who had a uterus and had undergone robotic supracervical hysterectomy with sacrocervicopexy (RSHS). The reported cure rate of the apical compartment was 91.4% for the RSC group and 99% for the RSHS group, while the cure rate for all compartments was 67.1% for the RSC group and 64.8% for the RSHS group. These results indicate that, in patients with a uterus, the surgical cure rate remains high. We prefer sacrohysteropexy if there is no contraindication for uterine preservation in patients with apical prolapse [27]. Hysteropexy has advantages of maintained fertility and natural menopausal timing due to preservation of the uterus, and 36%–60% of female patients would choose uterine preservation assuming equal surgical efficacy [29] (Table 2).
Comparative Studies
The clinical outcomes of RSC are comparable to those of open sacrocolpopexy (Table 2). Siddiqui et al. [20] retrospectively analyzed the outcomes of RSC (n=125) and open ASC (n=322) in patients with POP of stage 2 or higher after prior hysterectomy. An anatomic success rate of 94% was obtained for both groups over a 12-month follow-up period. In a logistic regression analysis that controlled for parity, concomitant hysterectomy, and posterior repair, no significant differences were found between RSC and ASC. Although the study was retrospective, the composite outcome was defined clearly; cases requiring repeat surgery due to bothersome vaginal bulge symptoms were defined as symptomatic failures, and cases of the vaginal apex descending to below the upper third of the vagina or anterior or posterior vaginal wall prolapse beyond the hymen were defined as anatomic failures. No significant differences were found in surgical failure based on composite outcomes of symptoms (RSC: 7 of 86 [8%] vs. LSC: 12 of 304 [4%]; P=0.16). In addition, RSC had the advantages of reduced blood loss during surgery and reduced hospital stay, although the operative time was longer than that of open sacrocolpopexy [8].
No difference in efficacy according to surgical method has been observed, even in comparison with LSC (Table 2). In 2011, Tan-Kim et al. [15] performed a retrospective analysis of 40 patients who had undergone RSC and 61 patients who had undergone LSC and found that the cure rate of the apical compartment was 100% in both groups during an average follow-up period of 6 months. There have been 2 randomized controlled trials (RCTs) of LSC and RSC since then. Paraiso et al. [13] conducted a 12-month follow-up observation after RSC (n=40) and LSC (n=38) in patients with POP-Q stage 2–4 after hysterectomy. The percentage of patients who achieved POP-Q stage 0–1 for the apical compartment was 88% in the RSC group and 91% in the LSC group, and this difference was not statistically significant. The authors reported that the operative time for RSC was significantly longer, and that RSC was associated with more severe pain than LSC with no cost benefit. In a 2016 RCT involving patients with symptomatic apical POP of POP-Q stage 2 and above, Kenton et al. [12] revealed in that both LSC and RSC patients showed significant improvement at the Ba, Bp, and C points during the 12-month follow-up period compared to before surgery. Illiano et al. [17] recently reported the outcomes of an RCT of RSC (n=49) and LSC (n=51) in patients with POP-Q stage 3–4. When cure was defined as prolapse stage <2 for all compartments, point C ≤-5, and total vaginal length of at least 7 cm, a 100% success rate for the apical compartment was reported in both groups during an average follow-up period of 24.1 months (Table 2).
Recurrence and Reoperation
It is challenging to define the success of POP repair. Is anatomical restoration to the original state a success? Is elimination of bulging symptoms felt by the patient a success? It is important to assess composite outcomes to address this issue. The importance of composite outcomes was clearly demonstrated by the CARE trial, which reported stratified outcomes as subjective, anatomic, or composite failure after POP repair. Anatomic failure after sacrocolpopexy was defined as postoperative POP requiring reoperation or pessary or recurrent prolapse according to the POP-Q system, defined as the vaginal apex descending to below the upper third of the vagina, or anterior or posterior vaginal wall prolapse beyond the hymen. Interestingly, half of the patients with anatomic failure at the 7-year follow-up reported no symptoms and did not require further treatment [30]. Culligan et al. [11] evaluated the clinical cure rate, as well as the conventional objective anatomic cure rate, in a prospective study of 143 RSC patients. Clinical cure was defined as (1) no reoperation for POP, (2) no symptoms of vaginal bulge, (3) no POP-Q point >0, (4) POP-Q point C ≤-5, and (5) an answer of “satisfied” or “very satisfied” on SSQ-8 (surgical satisfaction questionnaire 8). The clinical cure rate was 95% (136 of 143), higher than the anatomic cure rate of 84% based on the overall POP-Q stage. The authors further reported that 1 of the patients had a point C of -4, corresponding to clinical failure, but no POP symptoms, and was very satisfied with the outcomes of surgery. In 2 other patients, the POP-Q stage was restored to 0 or 1, but POP symptoms remained according to the pelvic floor distress inventory-20 questionnaire. In one of the largest prospective studies published by van Zanten et al. [21], the recurrence of apical prolapse was only 0.7% (1 of 140) for 12.6 months after RSC (n=188), but the recurrence rates for anterior compartment prolapse and posterior compartment prolapse were 15.7% (22 of 140) and 4.3% (6 of 140), respectively. However, reoperation was not required for symptomatic recurrence in 12.1% (17 of 140) of anterior compartment prolapse cases and 1.4% (2 of 140) of posterior compartment prolapse cases. Nevertheless, the reoperation rate of 8.3% for recurrence of anterior prolapse is still much higher than the rates for other compartments. In a long-term follow-up observational study conducted on 30 patients who were available for monitoring for more than 36 months after RSC, prolapse recurrence in the apical compartment was noted in 6.7% (2 of 30), but none of the patients required an additional operation. Among 13.3% of patients who needed a reoperation, 10% underwent anterior colporrhaphy, and 3.3% underwent posterior colporrhaphy [23] (Table 2).
Apical compartment prolapse occurs concomitantly with anterior and posterior compartment prolapse. We previously suggested that identification and correction of apical prolapse is critical to reduce recurrence after POP repair, and clinically significant apical prolapse is virtually always present in cases with both anterior and posterior compartment prolapse [29]. In most cases, the anterior and posterior compartments can be restored by apical repair alone. Nevertheless, recurrence of anterior or posterior compartment prolapse after sacrocolpopexy is due to the inability of the anterior and posterior vaginal walls to be dissected as far caudally as possible. Recurrence in the anterior compartment in the aforementioned RSC series is common, but the risk appears to be low compared to that of LSC. In a study of by Tan-Kim et al. [15], although there was no recurrence of apical prolapse after RSC and LSC, anterior compartment prolapse recurred in 2.5% of patients in the RSC group and 11% in the LSC group. Dissection of the vaginal wall up to the bladder neck appears to be challenging for novice surgeons performing LSC. The extended field of view and unlimited robot arm movement are important advantages for beginners with limited laparoscopic skills.
Operative Time and Endeavors to Reduce It
The reported mean or median operative time of RSC, defined as the time from incision to closure, varies widely, from a minimum of 124.2 minutes to a maximum of 288 minutes. An interesting result is that all 3 RCTs comparing RSC and LSC reported a longer operative time for RSC than for LSC [9,13,17]. In one prospective study, the operative time of RSC was 125 minutes (range, 90–270 minutes), significantly shorter than the 220 minutes required for LSC (range, 80–420 minutes) [19]. The surgeons who participated in the randomized trials were laparoscopic experts and appeared to have overcome the learning curve. Therefore, the operative time of LSC was shorter. However, robot-assisted laparoscopic surgery allows suturing and maneuvers requiring dexterity to be performed more quickly than does manual laparoscopy [31], and robotics can help novices overcome the learning curve rapidly [32]. Due to the nature of sacrocolpopexy, the most time-consuming procedure is suturing for mesh fixation and reperitonealization. Therefore, a novice in minimally invasive sacrocolpopexy may take less time to perform RSC than to master LSC.
When performing RSC, additional time is needed if concurrent vaginal surgery such as anterior or posterior repair and anti-incontinence surgery is required. However, except when antiincontinence surgery such as a sling is simultaneously needed, high-grade cystocele may be satisfactorily corrected by RSC alone, and the time for vaginal surgery can be reduced [33]. In patients with a uterus, uterine preservation may facilitate a shorter operative time than concurrent hysterectomy (supracervical or total) [34].
Mesh Complications and Lightweight Mesh
A systematic review of RSC studies reported mesh erosion rates ranging from 0% to 8% [8]. U.S. Food and Drug Administration recommendations and recent trends regarding use of mesh in POP repair restrict the use of transvaginal meshes [35]. Thus far, the use of mesh through a transabdominal approach does not appear to pose a problem. In a retrospective study that followed open sacrocolpopexy and RSC patients for an average of 1 year, the mesh erosion rate was 5.3% vs. 2.4%, indicating higher risk in the open sacrocolpopexy group, but without statistical significance [20]. In studies comparing LSC and RSC, mesh erosion rates of 0%–5% have been reported for patients undergoing RSC and 0%–6% for those undergoing LSC, suggesting that RSC does not reduce mesh complications. Fundamentally, the use of mesh through the abdominal approach in POP repair is associated with a significantly lower risk of meshrelated complications than the transvaginal approach. However, further long-term follow-up results are need to confirm this (Table 3).
Mesh materials have also been modified to reduce complications. Type 1 polypropylene mesh is the most commonly used material, although not all type 1 polypropylene materials are the same. In addition to mesh erosion, pain and dyspareunia can also be caused by mesh. To overcome these problems, lightweight or ultra-lightweight mesh products have been launched to reduce “mesh load.” A weight-based classification of mesh materials was introduced by Earle and Mark [36]: heavyweight (>80 g/m2), medium-weight (50–80 g/m2), and lightweight (<35 g/m2). A study comparing vaginal mesh extrusion rates between lightweight mesh and heavier mesh in patients with POP showed a 46% reduction in rate of mesh exposure in those receiving lighter-weight mesh, which may be of clinical importance [37]. Salamon et al. [14] conducted the first prospective study of an ultralight mesh, Restorelle Y Smartmesh (18.69 g/m2, Coloplast A/S, Humlebæk, Denmark), in RSC. The authors monitored 118 patients for 1 year and reported no mesh erosion or mesh-related complications. While the application of lighter mesh materials appears to be very promising, more research is needed. Other postoperative complications are summarized in Table 3.
CONCLUSIONS
Sacrocolpopexy through an abdominal approach is the current gold standard for the restoration of apical compartment prolapse. RSC is of interest at this time of transition from open surgery to minimally invasive surgery because of the increased risk of morbidity associated with open surgery. Good outcomes have been achieved with RSC even in advanced-stage POP, and there are data that indicate that this technique results in durable outcomes. In terms of postoperative morbidity and complications, RSC can greatly improve patients’ symptoms and quality of life. Particularly for novice surgeons who are not familiar with LSC, RSC is an excellent choice as the first surgical skill to attempt.
Notes
Conflict of Interest
The authors have no potential conflicts of interest relevant to this article.
AUTHOR CONTRIBUTION STATEMENT
·Conceptualization: KSL
·Formal Analysis: KJK
·Investigation: KJK, KSL
·Methodology: KJK
·Project Administration: KSL
·Writing – Original Draft: KJK
·Writing – Review & Editing: KSL
SUPPLEMENTARY MATERIALS
Supplementary video clip can be found via https://doi.org/10.5213/inj.2040056.028 or https://www.youtube.com/watch?v=5ksQBoCUQzc&t=13s.



